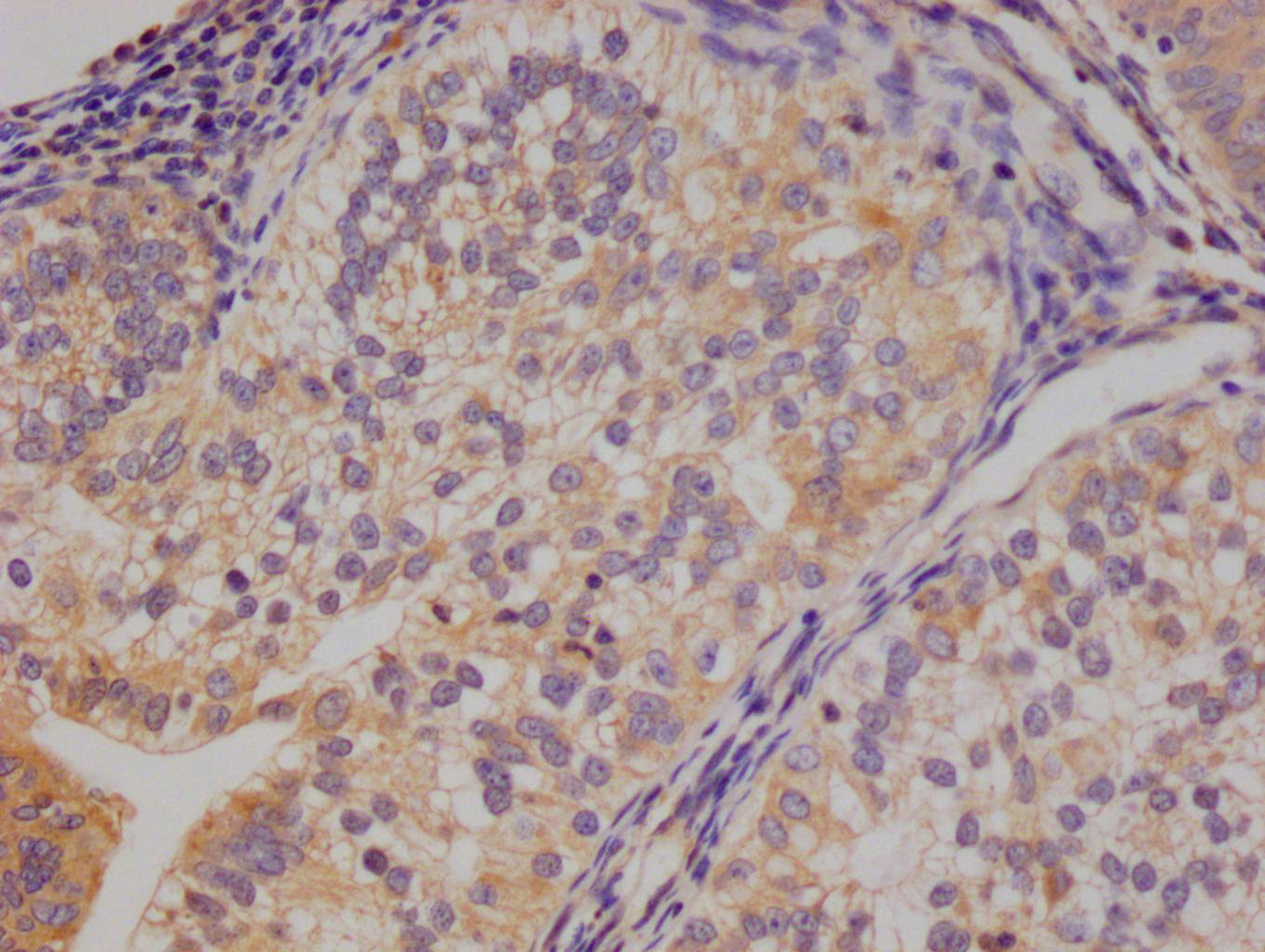
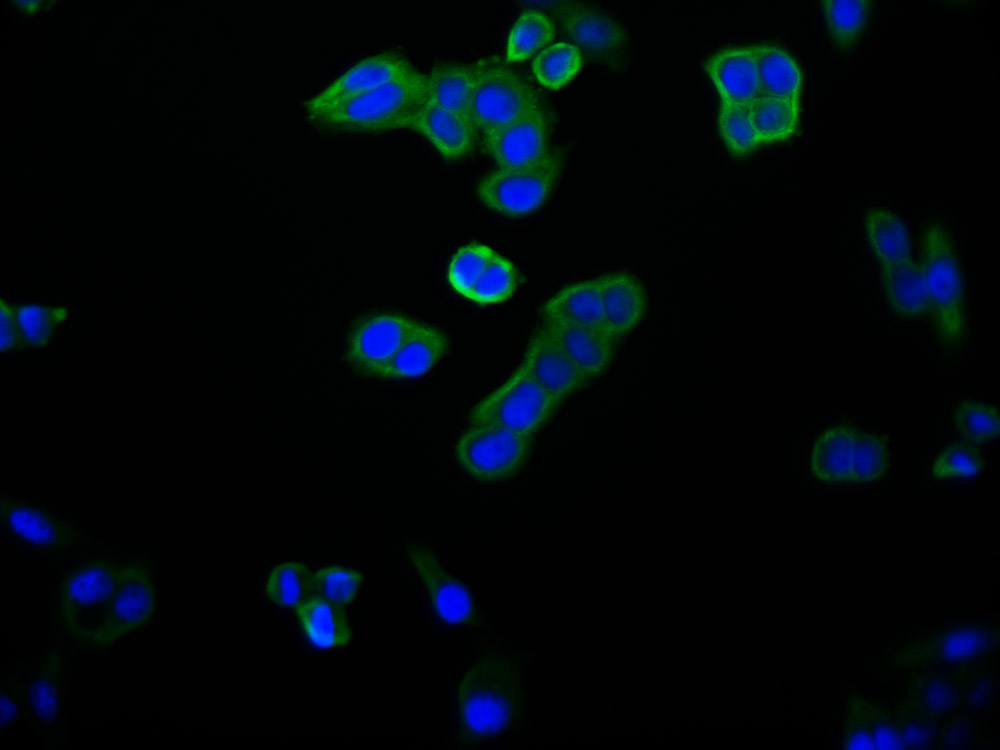
CDC37 Antibody
-
中文名稱:CDC37兔多克隆抗體
-
貨號:CSB-PA858597
-
規(guī)格:¥2024
-
圖片:
-
其他:
產(chǎn)品詳情
-
產(chǎn)品名稱:Rabbit anti-Homo sapiens (Human) CDC37 Polyclonal antibody
-
Uniprot No.:
-
基因名:
-
宿主:Rabbit
-
反應(yīng)種屬:Human,Mouse,Rat
-
免疫原:Synthesized peptide derived from internal of Human p50 CDC37.
-
免疫原種屬:Homo sapiens (Human)
-
克隆類型:Polyclonal
-
純化方式:The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
-
濃度:It differs from different batches. Please contact us to confirm it.
-
產(chǎn)品提供形式:Liquid
-
應(yīng)用范圍:ELISA,WB
-
推薦稀釋比:
Application Recommended Dilution WB 1:500-1:3000 -
Protocols:
-
儲存條件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
貨期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相關(guān)產(chǎn)品
靶點詳情
-
功能:Co-chaperone that binds to numerous kinases and promotes their interaction with the Hsp90 complex, resulting in stabilization and promotion of their activity. Inhibits HSP90AA1 ATPase activity.
-
基因功能參考文獻(xiàn):
- Study showed that Cdc37 gene was up-regulated in human colorectal adenocarcinoma (CRC). Furthermore, knockdown of Cdc37 effectively reduced cell proliferation activity, enhanced apoptosis, and inhibited G1-S transition in CRC cells, and vice versa. For the mechanism, Cdc37 increased CDK4 stability to promote the phosphorylation of RB1, which finally promoted the progression of CRC. PMID: 29288563
- During the kinase chaperone cycle, Cdc37 phosphorylated at Y298 acts as a platform for docking of non-receptor tyrosine kinases through their regulatory domains to drive the coupled Hsp90 phosphorylation at Y197 and specifically regulate kinase chaperoning. PMID: 29343704
- findings suggested that this mechanism may be exploited by the Hsp90-Cdc37 chaperone to recruit and protect intrinsically dynamic kinase clients from degradation PMID: 29267381
- The results suggest a re-evaluation of the role of Cdc37 in the kinase lifecycle, and suggest that such interactions potentially allow kinases to more rapidly respond to key signals while simultaneously protecting unstable kinases from degradation and suppressing unwanted basal activity. PMID: 28784328
- Niclosamide ethanolamine disrupted the interaction between cell division cycle 37 and heat shock protein 90 in hepatocellular carcinoma, reducing tumor growth. PMID: 28284560
- Cdc37 performs a quality control of protein kinases, including b-raf, where induced conformational instability acts as a "flag" for Hsp90 dependence and stable cochaperone association. PMID: 27105117
- Ulk1 promoted the degradation of Hsp90-Cdc37 client kinases, resulting in increased cellular sensitivity to Hsp90 inhibitors. Thus, our study provides evidence for an anti-proliferative role of Ulk1 in response to Hsp90 inhibition in cancer cells PMID: 28073914
- The authors find that the interaction between sB-Raf and the Hsp90 chaperone system is based on contacts with the M domain of Hsp90, which contributes in forming the ternary complex with Cdc37 as long as the kinase is not stabilized by nucleotide. PMID: 27620500
- Apart from these distinct Cdc37/Hsp90 interfaces, binding of the B-Raf protein kinase to the cochaperone is conserved between mammals and nematodes. PMID: 26511315
- Suppressing expression of the cochaperone CDC37 in hepatocellular carcinoma cells inhibits cell cycle progression and cell growth. PMID: 25098386
- Correlation between PDZK1, Cdc37, Akt and breast cancer malignancy: the role of PDZK1 in cell growth through Akt stabilization by increasing and interacting with Cdc37 PMID: 24869908
- The N-terminal tail serves as an intramolecular chaperone ensuring that CDC37 assumes one of two interconvertible states in a manner impacting the interaction of the client binding N-domain and the MC-domains, involved in dimerization and HSP90 binding. PMID: 25619116
- CDC37 has an important role in chaperoning protein kinases; it stabilizes kinase clients by a mechanism that is not dependent on a substantial direct interaction between CDC37 and HSP90, but requires HSP90 activity PMID: 24292678
- As a novel Hsp90 inhibitor, FW-04-806 binds to the N-terminal of Hsp90 and inhibits Hsp90/Cdc37 interaction, resulting in the disassociation of Hsp90/Cdc37/client complexes and the degradation of Hsp90 client proteins. PMID: 24927996
- CDC37 is a crucial HSP90-cofactor for KIT oncogenic expression in gastrointestinal stromal tumors PMID: 23584476
- SGK3 stability and kinase activation are regulated by the Hsp90-Cdc37 chaperone complex. PMID: 24379398
- Cdc37 (cell division cycle 37) restricts Hsp90 (heat shock protein 90) motility by interaction with N-terminal and middle domain binding sites. PMID: 23569206
- ERK5 interacts with the Hsp90-Cdc37 chaperone in resting cells, and inhibition of Hsp90 or Cdc37 results in ERK5 ubiquitylation and proteasomal degradation. PMID: 23428871
- an essential role for surface Cdc37 in concert with HSP90 on the cell surface during cancer cell invasion processes PMID: 22912728
- A series of tyrosine phosphorylation events, involving both p50(Cdc37) and Hsp90, are minimally sufficient to provide directionality to the chaperone cycle. PMID: 22727666
- Data show that part of the normal clearance cascade for TDP-43 involves the Cdc37/Hsp90 complex. PMID: 22674575
- Cdc37-mediated direct interaction between Hsp90/Cdc37 and an IRE1alpha cytosolic motif is important to maintain basal IRE1alpha activity and contributes to normal protein homeostasis and unfolded protein response under physiological stimulation. PMID: 22199355
- The primary mechanisms by which apigenin kill multiple myeloma cells is by targeting the trinity of CK2-Cdc37-Hsp90. PMID: 21871133
- the Hsp90 kinase co-chaperone Cdc37 regulates tau stability and phosphorylation dynamics PMID: 21367866
- Hsp90-Cdc37 complex acta as an endogenous regulator of noncanonical p38alpha activity. PMID: 20299663
- Tnf-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. PMID: 11864612
- role in regulating Hsp90 ATPase activity PMID: 11916974
- CDC37 binds to Akt and HSP90 in the signal transduction pathway in human tumor cells PMID: 12176997
- Results show that Cdc37 and heat shock protein 90 bind specifically to the kinase domain of LKB1. PMID: 12489981
- phosphorylation of Cdc37 on Ser13 is critical for its ability to coordinate Hsp90 nucleotide-mediated conformational switching and kinase binding PMID: 12930845
- Heteromeric comlpexes containing the molecular chaperones Hsp90 and Cdc37/p50 interacts with the kinase domain of LKB1. PMID: 14668798
- the interaction of Cdc37 with its client protein kinases requires amino acid residues within a motif that is present in many protein kinases PMID: 14701845
- Hsp90/p50cdc37 is required for mixed-lineage kinase (MLK) 3 signaling PMID: 15001580
- the Hsp90.Cdc37 molecular chaperone module has a central role in interleukin-1 receptor-associated-kinase-dependent signaling by toll-like receptors PMID: 15647277
- Cdc37 is found to heterodimerize with heat-shock protein 90 (Hsp90)-associating relative of Cdc37 (Harc) in vitro. PMID: 15850399
- Nuclear magnetic resonance study of binding to to HSP90. PMID: 16132836
- results suggest that a region of Cdc37 other than the client-binding site may be responsible for discriminating client protein kinases from others PMID: 16156789
- JAK1/2 are client proteins of Hsp90 alpha and beta; Hsp90 and CDC37 play a critical role in types I and II interferon pathways PMID: 16280321
- N-terminal glycine-rich loop of protein kinases is essential for physically associating with Cdc37. PMID: 16611982
- The data shows the expression and purification of an Hsp90-Cdc37-Cdk4 complex, defining its stoichiometry, and determining its 3D structure by single-particle electron microscopy. PMID: 16949366
- these observations support the hypothesis that there is a specific coordination between the activation of the cytosolic Ah receptor and the c-Src- and cdc37-containing HSP90 complex. PMID: 17223712
- The present data denote Hsp90-Cdc37 as a transiently acting essential regulatory component of IKK signaling. PMID: 17728246
- identify Pink1 as a novel Cdc37/Hsp90 client kinase PMID: 18003639
- Cdc37 is essential for maintaining prostate tumor cell growth and may represent a novel target in the search for multitargeted therapies. PMID: 18089825
- These data reveal a cyclic regulatory mechanism for Cdc37, in which its constitutive phosphorylation is reversed by targeted dephosphorylation in Hsp90 complexes. PMID: 18922470
- CDC37 in concert with HSP90 plays an essential role in maintaining oncogenic protein kinase clients including ERBB2, CRAF, CDK4, CDK6, & phosphorylated AKT. PMID: 18931700
- The human Cdc37.Hsp90 complex studied by heteronuclear NMR spectroscopy. PMID: 19073599
- C-terminal tail and determinants in the alphaE-helix of the catalytic domain allows the chaperones Hsp90 and Cdc37 to bind newly synthesized PKC beta II, a required event in the processing of PKC by phosphorylation PMID: 19091746
- celastrol may represent a new class of Hsp90 inhibitor by modifying Hsp90 C terminus to allosterically regulate its chaperone activity and disrupt Hsp90-Cdc37 complex. PMID: 19858214
顯示更多
收起更多
-
亞細(xì)胞定位:Cytoplasm.
-
蛋白家族:CDC37 family
-
數(shù)據(jù)庫鏈接:
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-