ERO1L Antibody
-
中文名稱:ERO1L兔多克隆抗體
-
貨號:CSB-PA007797GA01HU
-
規(guī)格:¥3,900
-
其他:
產(chǎn)品詳情
-
Uniprot No.:
-
基因名:
-
別名:Endoplasmic oxidoreductin 1 like protein antibody; Endoplasmic oxidoreductin-1-like protein antibody; Endoplasmic reticulum oxidoreductase 1 alpha antibody; Endoplasmic reticulum oxidoreductin 1-like antibody; ERO1 alpha antibody; ERO1 L antibody; ERO1 Lalpha antibody; ERO1 like protein alpha antibody; ERO1-alpha antibody; ERO1-L antibody; ERO1-L-alpha antibody; ERO1-like (S. cerevisiae) antibody; ERO1-like alpha antibody; ERO1-like protein alpha antibody; ERO1-like; S. cerevisiae; homolog of; alpha antibody; ERO1A antibody; ERO1A_HUMAN antibody; ERO1L antibody; ERO1LA antibody; Oxidoreductin 1 Lalpha antibody; Oxidoreductin-1-L-alpha antibody; PRO865 antibody; UNQ434 antibody
-
宿主:Rabbit
-
反應種屬:Human,Mouse
-
免疫原:Human ERO1L
-
免疫原種屬:Homo sapiens (Human)
-
抗體亞型:IgG
-
純化方式:Antigen Affinity purified
-
濃度:It differs from different batches. Please contact us to confirm it.
-
保存緩沖液:PBS with 0.02% Sodium Azide, 50% Glycerol, pH 7.3. -20°C, Avoid freeze / thaw cycles.
-
產(chǎn)品提供形式:Liquid
-
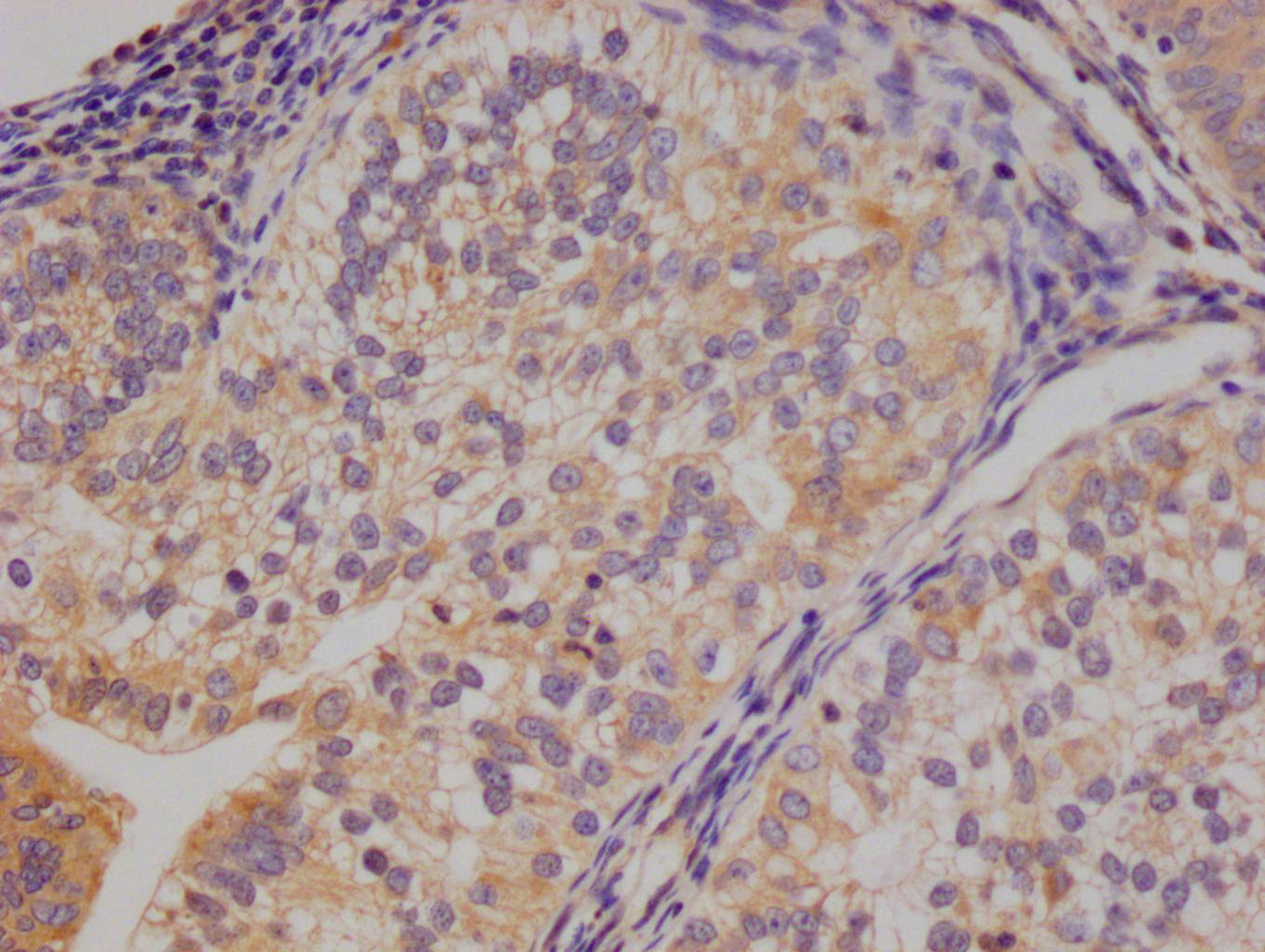
應用范圍:ELISA,IHC
-
Protocols:
-
儲存條件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
貨期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相關產(chǎn)品
靶點詳情
-
功能:Oxidoreductase involved in disulfide bond formation in the endoplasmic reticulum. Efficiently reoxidizes P4HB/PDI, the enzyme catalyzing protein disulfide formation, in order to allow P4HB to sustain additional rounds of disulfide formation. Following P4HB reoxidation, passes its electrons to molecular oxygen via FAD, leading to the production of reactive oxygen species (ROS) in the cell. Required for the proper folding of immunoglobulins. Involved in the release of the unfolded cholera toxin from reduced P4HB/PDI in case of infection by V.cholerae, thereby playing a role in retrotranslocation of the toxin. Plays an important role in ER stress-induced, CHOP-dependent apoptosis by activating the inositol 1,4,5-trisphosphate receptor IP3R1.
-
基因功能參考文獻:
- High expression of ERO1L is associated with poor prognosis of patients with gastric cancer. These results indicate that ERO1L expression may be a clinically promising therapeutic target for prevention of gastric cancer. PMID: 26987398
- ERO1alpha plays a crucial role in HSC proliferation via posttranslational modification of collagen and MT1-MMP PMID: 28774960
- Study shows that ERO1L and NARS expression level are up-regulated in primary lung adenocarcinoma and identifies them with a potential to promote tumor metastasis and growth of cancer cells. PMID: 27161446
- a mechanism of dual Ero1alpha regulation by dynamic redox interactions between PDI and the two Ero1alpha flexible loops that harbor the regulatory cysteines. PMID: 27703014
- Expression of ERO1-alpha in MDA-MB-231 cells promotes tumour growth via promoting angiogenesis. Knockdown of ERO1-alpha inhibits secretion of VEGF by inhibition of oxidative protein folding. In triple-negative breast cancer cases, the expression of ERO1-alpha was upregulated and related to the number of the blood vessels. ERO1-alpha was a poor prognosis factor in triple-negative breast cancer. PMID: 27100727
- Data indicate a new mechanism of Ero1alpha regulation in which thiol-disulfide exchange at Cys208-Cys241 affects the stability of the Cys94-Cys131 inhibitory disulfide through allosteric and/or inter-molecular communication. PMID: 26609561
- the cancer-associated ERO1-alpha regulates the expression of the MHC class I molecule via oxidative folding PMID: 25870246
- Data suggest that expression of ERO1alpha (oxidoreductin-1-L-alpha) and CHOP (c/EBP-homologous protein) is up-regulated in liver of patients with acute liver failure. PMID: 25387528
- These results suggest that overexpression of ERO1-alpha in the tumor inhibits the T cell response by recruiting polymorphonuclear myeloid-derived suppressor cells PMID: 25595776
- PDI has a role as a competent regulator and a specific substrate of Ero1alpha govern efficient and faithful oxidative protein folding and maintain the ER redox homeostasis PMID: 25258311
- These results indicate that BPA, a widely distributed and potentially harmful chemical, inhibits Ero1-PDI-mediated disulfide bond formation. PMID: 25122773
- The high expression of Ero1alpha in cancers of the esophagus and stomach demonstrates the importance of ER redox regulation in the gastro-intestinal (GI) tract in health and disease. PMID: 23373818
- GPx7 promotes oxidative protein folding, directly utilizing Ero1alpha-generated hydrogen peroxide in the early secretory compartment. PMID: 23919619
- A simple feedback mechanism of regulation of Ero1alpha was identified involving its primary substrate. PMID: 24758166
- The expression of hERO1-alpha in cancer cells is associated with poorer prognosis. PMID: 23578220
- Report on the establishment of an engineered CHOS cell line that has been engineered to express both XBP-1S) and ERO1-Lalpha and has been named CHOS-XE. CHOS-XE cells produced increased antibody (MAb) yields (5.3- 6.2 fold) in comparison to CHOS cells. PMID: 23335490
- ER stress induced by misfolded proinsulin was limited by increased expression of Ero1alpha, suggesting that enhancing the oxidative folding of proinsulin may be a viable therapeutic strategy in the treatment of type 2 diabetes. PMID: 24022479
- This paper reported the interactions of Ero1 with protein disulfide isomerase family proteins and chaperones, highlighting the effect that redox flux has on Ero1 partnerships. PMID: 23530257
- hyperoxidation generated by Ero1alpha-C104A/C131A is addressed in the ER lumen and is unlikely to exert oxidative injury throughout the cell. PMID: 23027870
- the levels, subcellular localization, and activity of Ero1alpha coordinately regulate Ca(2+) and redox homeostasis and signaling in the early secretory compartment. PMID: 21854214
- the intramolecular electron transfer from the a domain to the a' domain within PDI during its oxidation by ERO1alpha. PMID: 21757736
- Molecular bases of cyclic and specific disulfide interchange between human ERO1alpha protein and protein-disulfide isomerase (PDI). PMID: 21398518
- Results show that within the ER, Ero1alpha is almost exclusively found on the mitochondria-associated membrane (MAM). PMID: 20186508
- A dynamic equilibrium between Ero1- and glutathione disulphide-mediated oxidation of protein disulphide isomerases constitutes an important element of endoplasmic reticulum redox homeostasis. PMID: 20802462
- Ero1alpha limits its oxidative activity using four regulatory cysteines properly positioned within a critical loop that transfers electrons from protein disulphide isomerase to the FAD-containing active site. PMID: 20834232
- proving that the activity of Ero1-Lalpha results in H(2)O(2) formation in the endoplasmic reticulum PMID: 20095866
- Ero1alpha is expressed on blood platelets in association with protein-disulfide isomerase and contributes to redox-controlled remodeling of alphaIIbbeta3. PMID: 20562109
- The results demonstrate that the specificity of Ero1alpha toward the active sites of PDI requires the presence of the regulatory disulfides. PMID: 20657012
- an appropriate Ero1alpha-PDI ratio is critical for regulating the binding-release cycle of CTA1 by PDI during retro-translocation, and PDI's redox state has a role in targeting it to the retro-translocon PMID: 20130085
- oxygen regulation of ERO1-Lalpha expression likely is to maintain the transfer rate of oxidizing equivalents to PDI in situations of an altered cellular redox state induced by changes of the cellular oxygen tension PMID: 12752442
- two conserved cysteine triads in human Ero1alpha cooperate for efficient disulfide bond formation in the endoplasmic reticulum PMID: 15136577
- glutathione limits Ero1-dependent oxidation in the endoplasmic reticulum PMID: 15161913
- Hypoxic induction of Ero1-L alpha is key adaptive response in a previously unrecognized HIF-1-mediated pathway that operates to improve protein secretion under hypoxia and might be used for inhibiting tumor growth via inhibiting VEGF-driven angiogenesis. PMID: 15592500
- Results determine the redox-driven shutdown mechanism of Ero1alpha from the formation of a disulphide bond between the active-site Cys(94) and Cys(131). PMID: 18833192
- Data suggest that partial regulatory disulfide reduction may be a mechanism for preventing excessive Ero1alpha activity and oxidation of PDI or that additional factors are required for Ero1alpha activation within the mammalian ER. PMID: 18971943
- In this report, the structural model of human Ero1-L alpha was built at first. PMID: 19766098
顯示更多
收起更多
-
亞細胞定位:Endoplasmic reticulum membrane; Peripheral membrane protein; Lumenal side. Note=The association with ERP44 is essential for its retention in the endoplasmic reticulum.
-
蛋白家族:EROs family
-
組織特異性:Widely expressed at low level. Expressed at high level in upper digestive tract. Highly expressed in esophagus. Weakly expressed in stomach and duodenum.
-
數(shù)據(jù)庫鏈接:
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-