HSP90AB1 Recombinant Monoclonal Antibody
-
中文名稱:HSP90AB1重組抗體
-
貨號(hào):CSB-RA904894A0HU
-
規(guī)格:¥1320
-
圖片:
-
Western Blot
Positive WB detected in: HepG2 whole cell lysate, U251 whole cell lysate, MCF-7 whole cell lysate, A549 whole cell lysate, HEK293 whole cell lysate, Hela whole cell lysate
All lanes: HSP90AB1 antibody at 1:2000
Secondary
Goat polyclonal to rabbit IgG at 1/50000 dilution
Predicted band size: 84 kDa
Observed band size: 80-90 kDa -
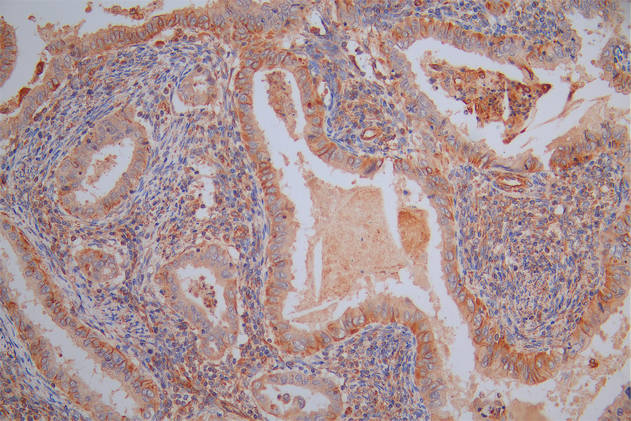
IHC image of CSB-RA904894A0HU diluted at 1:100 and staining in paraffin-embedded human testis tissue performed on a Leica BondTM system. After dewaxing and hydration, antigen retrieval was mediated by high pressure in a citrate buffer (pH 6.0). Section was blocked with 10% normal goat serum 30min at RT. Then primary antibody (1% BSA) was incubated at 4°C overnight. The primary is detected by a Goat anti-rabbit polymer IgG labeled by HRP and visualized using 0.05% DAB.
-
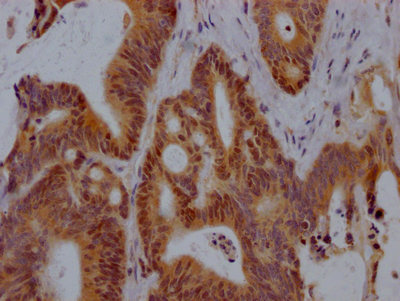
IHC image of CSB-RA904894A0HU diluted at 1:100 and staining in paraffin-embedded human colon tissue performed on a Leica BondTM system. After dewaxing and hydration, antigen retrieval was mediated by high pressure in a citrate buffer (pH 6.0). Section was blocked with 10% normal goat serum 30min at RT. Then primary antibody (1% BSA) was incubated at 4°C overnight. The primary is detected by a Goat anti-rabbit polymer IgG labeled by HRP and visualized using 0.05% DAB.
-
Immunofluorescence staining of HepG2 cell with CSB-RA904894A0HU at 1:50, counter-stained with DAPI. The cells were fixed in 4% formaldehyde and blocked in 10% normal Goat Serum. The cells were then incubated with the antibody overnight at 4°C. The secondary antibody was Alexa Fluor 495-congugated AffiniPure Goat Anti-Rabbit IgG(H+L).
-
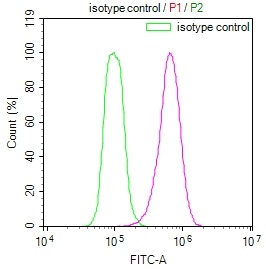
Overlay Peak curve showing Hela cells stained with CSB-RA904894A0HU (red line) at 1:100. The cells were fixed in 4% formaldehyde and permeated by 0.2% TritonX-100. Then 10% normal goat serum to block non-specific protein-protein interactions followed by the antibody (1ug/1*106cells) for 45min at 4℃. The secondary antibody used was FITC-conjugated Goat Anti-rabbit IgG(H+L) at 1:200 dilution for 35min at 4℃.Control antibody (green line) was rabbit IgG (1ug/1*106cells) used under the same conditions. Acquisition of >10,000 events was performed.
-
-
其他:
產(chǎn)品詳情
-
Uniprot No.:
-
基因名:
-
別名:Heat shock protein HSP 90-beta (HSP 90) (Heat shock 84 kDa) (HSP 84) (HSP84), HSP90AB1, HSP90B HSPC2 HSPCB
-
反應(yīng)種屬:Human
-
免疫原:A synthesized peptide derived from human HSP90AB1
-
免疫原種屬:Homo sapiens (Human)
-
標(biāo)記方式:Non-conjugated
-
克隆類型:Monoclonal
-
抗體亞型:Rabbit IgG
-
純化方式:Affinity-chromatography
-
克隆號(hào):7G7
-
濃度:It differs from different batches. Please contact us to confirm it.
-
保存緩沖液:Rabbit IgG in phosphate buffered saline, pH 7.4, 150mM NaCl, 0.02% sodium azide and 50% glycerol.
-
產(chǎn)品提供形式:Liquid
-
應(yīng)用范圍:ELISA, WB, IHC, IF, FC
-
推薦稀釋比:
Application Recommended Dilution WB 1:500-1:2000 IHC 1:50-1:200 IF 1:50-1:200 FC 1:50-1:200 -
Protocols:
-
儲(chǔ)存條件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
貨期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相關(guān)產(chǎn)品
靶點(diǎn)詳情
-
功能:Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle linked to its ATPase activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function. Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself. Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co-chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle. Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels. They first alter the steady-state levels of certain transcription factors in response to various physiological cues. Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment. Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression. Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation. Promotes cell differentiation by chaperoning BIRC2 and thereby protecting from auto-ubiquitination and degradation by the proteasomal machinery. Main chaperone involved in the phosphorylation/activation of the STAT1 by chaperoning both JAK2 and PRKCE under heat shock and in turn, activates its own transcription. Involved in the translocation into ERGIC (endoplasmic reticulum-Golgi intermediate compartment) of leaderless cargos (lacking the secretion signal sequence) such as the interleukin 1/IL-1; the translocation process is mediated by the cargo receptor TMED10.
-
基因功能參考文獻(xiàn):
- The production of IFN-gamma by T cells stimulated with citrullinated HSP90beta demonstrates a bias toward TH1 immune responses that are likely involved in the pathogenesis of rheumatoid arthritis-interstitial lung disease. PMID: 29968330
- the expressions of HSP90AB1 can predict prognosis in astrocytic tumors PMID: 27258564
- We found that the nutrient value of the culturing medium and the length of induction had significant effect on Hsp90 production in Escherichia coli. Our fast, single-day purification protocol resulted in a stable, well-folded and pure sample that was resistant to degradation in a reproducible manner. PMID: 28651008
- Data show that C allele of rs2282151 was associated with increased expression level of heat shock protein 90 alpha family class B member 1 (HSP90AB1). PMID: 27756247
- Hsp90beta induced endothelial cell-dependent tumor angiogenesis by activating VEGFRs transcription. PMID: 28359326
- The authors find that the interaction between sB-Raf and the Hsp90 chaperone system is based on contacts with the M domain of Hsp90, which contributes in forming the ternary complex with Cdc37 as long as the kinase is not stabilized by nucleotide. PMID: 27620500
- High HSP90B expression is associated with laryngeal carcinoma. PMID: 27959448
- The expression level of Hsp90AB1 in lung cancer tissues was significantly higher than that in normal lung tissue and was associated with lung cancer pathological type and overall survival in lung adenocarcinoma patients. PMID: 26903158
- Apart from these distinct Cdc37/Hsp90 interfaces, binding of the B-Raf protein kinase to the cochaperone is conserved between mammals and nematodes. PMID: 26511315
- HSP90AB1: Helping the good and the bad PMID: 26358502
- These results suggest a means by which the hsp90beta interaction could prevent apo-sGCbeta1 from associating with its partner sGCalpha1 subunit while enabling structural changes to assist heme insertion into the H-NOX domain. PMID: 26134567
- Casein kinase 2-mediated phosphorylation of Hsp90beta and stabilization of PXR is a key mechanism in the regulation of MDR1 expression. PMID: 25995454
- This study identifies overexpression of HSP90 (especially isoform HSP90AB1) and its clients ATR, ATM, and NBS1 as promising markers for radioresistant, aggressive soft tissue sarcomas with particularly poor prognosis. PMID: 26044951
- The proteins (HSP90b, TMS1 and L-plastin) in the current study may hold potential in differentiating between melanoma and benign nevi in diagnostically challenging cases. PMID: 25191796
- The expression levels of Hsp90-beta and annexin A1 positively correlated and such co-overexpression of Hsp90-beta and annexin A1 contributed to lung cancer diagnosis. PMID: 25300907
- Hsp90 binds directly to fibronectin (FN) and inhibition reduces the extracellular fibronectin matrix in breast cancer cells. PMID: 24466266
- A novel mechanism for human carcinogenesis via methylation of HSP90AB1 by SMYD2. PMID: 24880080
- Hsp90 is upregulated in systemic sclerosis (SSc) and is critical for TGF-beta signalling. PMID: 23661493
- HSP70 was massively up-regulated in all mast cells three months after irradiation whereas HSP90AB1 was up-regulated only in a portion of mast cells PMID: 24670792
- Study obtained a structural model of Hsp90 in complex with its natural disease-associated substrate, the intrinsically disordered Tau protein. Hsp90 binds to a broad region in Tau that includes the aggregation-prone repeats. PMID: 24581495
- HSP90beta may positively regulate angiogenesis, not only as a protein chaperone, but also as an mRNA stabilizer for pro-angiogenic genes, such as BAZF, in a PRKD2 activity-dependent manner. PMID: 23515950
- Here we describe the specific association of heat shock protein-90-beta (Hsp90beta) with EV71 viral particles by the co-purification with virions using sucrose density gradient ultracentrifugation and colocalization as shown by immunogold EM. PMID: 23711381
- differences in expression caused by the -144 polymorphism in the HSP90beta promoter are associated with cellular inflammatory responses and the severity of organ injury PMID: 23516526
- the transdominant effect of HSP90AB1 on capsid-spacer protein 1-mutant HIV infectivity suggests a potential role for this class of cellular chaperones in HIV core stability and uncoating. PMID: 23200770
- The upregulation of Hsp90-beta was associated with poor post-surgical survival time and lymphatic metastasis of lung cancer patients PMID: 22929401
- DNA sequencing of 101 human samples detects eight and seven unique single nucleotide polymorphisms (SNPs) at the HSP90AA1 and HSP90AB1 loci, respectively. PMID: 22185817
- a possible role for HSP90AB1 in postentry HIV replication and may provide an attractive target for therapeutic intervention. PMID: 21602280
- TRIM8 modulates translocation of phosphorylated STAT3 into the nucleus through interaction with Hsp90beta and consequently regulates transcription of Nanog in embryonic stem cells. PMID: 21689689
- cyclophilin A and Hsp90 facilitate translocation of lethal factor(N) diphtheria toxin, but not of lethal factor, across endosomal membranes, and thus they function selectively in promoting translocation of certain proteins, but not of others PMID: 20946244
- Results show that RPL4, RPLP0, and HSPCB were the most stable reference genes in ovarian tissues. PMID: 20705598
- H. pylori induces the translocation of HSP90beta from the cytosol to the membrane and interaction of HSP90beta and Rac1, which leads to the activation of NADPH oxidase and production of ROS in gastric epithelial cells. PMID: 20451655
- findings present novel Hsp90 mutants that render cells resistant to Hsp90 inhibitors; show that the resistance depends on the increased ATPase turnover due to enhanced interaction with Aha1 PMID: 20226818
- Data show that the small molecule celastrol inhibits the Hsp90 chaperoning machinery by inactivating the co-chaperone p23, resulting in a more selective destabilization of steroid receptors. PMID: 19996313
- Study points to a potential role for Hsp90beta in MSC biology. PMID: 19327008
- PKC-epsilon is specifically required in the signaling pathway leading to the induction of hsp90 beta gene in response to heat shock. PMID: 14532285
- hsp90beta is repressed by p53 in UV irradiation-induced apoptosis PMID: 15284248
- Mutations at the phosphorylation sites of HSP90-beta modulate the interaction with arylhydrocarbon receptor (AhR) and may negatively regulate formation of the functional AhR complex in the steady-state cytosol. PMID: 15581363
- Hydrogen-exchange mass spectrometry was used to study structural & conformational changes undergone by full-length Hsp90beta in solution upon binding of the co-chaperone Cdc37 & 2 Hsp90 ATPase inhibitors: Radicicol & the anticancer drug DMAG PMID: 17764690
- Results suggest that HSP90 beta prevents auto-ubiquitination and degradation of its client protein c-IAP1, whose depletion would be sufficient to inhibit cell differentiation. PMID: 18239673
- These data provide an explanation for apoptosome inhibition by activated leukemogenic tyrosine kinases and suggest that alterations in Hsp90beta-apoptosome interactions may contribute to chemoresistance in leukemias. PMID: 18591256
- Presence of ovarian autoantibodies to human HSP90 in sera of women with infertility could be involved in human ovarian autoimmunity and thereby be a causative factor in early ovarian failure. PMID: 19022436
- Results show that heat shock protein 90 beta is cleaved by activated caspase-10 under UVB irradiation. PMID: 19380486
- celastrol may represent a new class of Hsp90 inhibitor by modifying Hsp90 C terminus to allosterically regulate its chaperone activity and disrupt Hsp90-Cdc37 complex. PMID: 19858214
顯示更多
收起更多
-
亞細(xì)胞定位:Cytoplasm. Melanosome. Nucleus. Secreted. Cell membrane. Dynein axonemal particle.
-
蛋白家族:Heat shock protein 90 family
-
數(shù)據(jù)庫(kù)鏈接:
Most popular with customers
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-
-
-