Recombinant Escherichia coli 60 kDa chaperonin (groL)
-
中文名稱:大腸桿菌groL重組蛋白
-
貨號(hào):CSB-YP363853ENV
-
規(guī)格:
-
來源:Yeast
-
其他:
-
中文名稱:大腸桿菌groL重組蛋白
-
貨號(hào):CSB-EP363853ENV
-
規(guī)格:
-
來源:E.coli
-
中文名稱:大腸桿菌groL重組蛋白
-
貨號(hào):CSB-EP363853ENV-B
-
規(guī)格:
-
來源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:大腸桿菌groL重組蛋白
-
貨號(hào):CSB-BP363853ENV
-
規(guī)格:
-
來源:Baculovirus
-
其他:
-
中文名稱:大腸桿菌groL重組蛋白
-
貨號(hào):CSB-MP363853ENV
-
規(guī)格:
-
來源:Mammalian cell
-
其他:
產(chǎn)品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:groL
-
Uniprot No.:
-
別名:groL; groEL; mopA; b4143; JW410360 kDa chaperonin; GroEL protein; Protein Cpn60
-
種屬:Escherichia coli (strain K12)
-
蛋白長度:Full Length of Mature Protein
-
表達(dá)區(qū)域:2-548
-
氨基酸序列AAKDVKFGN DARVKMLRGV NVLADAVKVT LGPKGRNVVL DKSFGAPTIT KDGVSVAREI ELEDKFENMG AQMVKEVASK ANDAAGDGTT TATVLAQAII TEGLKAVAAG MNPMDLKRGI DKAVTAAVEE LKALSVPCSD SKAIAQVGTI SANSDETVGK LIAEAMDKVG KEGVITVEDG TGLQDELDVV EGMQFDRGYL SPYFINKPET GAVELESPFI LLADKKISNI REMLPVLEAV AKAGKPLLII AEDVEGEALA TLVVNTMRGI VKVAAVKAPG FGDRRKAMLQ DIATLTGGTV ISEEIGMELE KATLEDLGQA KRVVINKDTT TIIDGVGEEA AIQGRVAQIR QQIEEATSDY DREKLQERVA KLAGGVAVIK VGAATEVEMK EKKARVEDAL HATRAAVEEG VVAGGGVALI RVASKLADLR GQNEDQNVGI KVALRAMEAP LRQIVLNCGE EPSVVANTVK GGDGNYGYNA ATEEYGNMID MGILDPTKVT RSALQYAASV AGLMITTECM VTDLPKNDAA DLGAAGGMGG MGGMGGMM
-
蛋白標(biāo)簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產(chǎn)品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復(fù)溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲(chǔ)存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項(xiàng):Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶點(diǎn)詳情
-
功能:Together with its co-chaperonin GroES, plays an essential role in assisting protein folding. The GroEL-GroES system forms a nano-cage that allows encapsulation of the non-native substrate proteins and provides a physical environment optimized to promote and accelerate protein folding, probably by preventing aggregation and by entropically destabilizing folding intermediates. Rapid binding of ATP, followed by slower binding of the non-native substrate protein and GroES to the cis open ring of GroEL initiates productive folding of the non-native protein inside a highly stable GroEL-ATP-GroES complex. Binding of ATP and GroES induces conformational changes that result in the release of the substrate protein into a nano-cage compartment, within the GroEL central cavity, for folding in isolation. To discharge GroES and substrate protein, ATP hydrolysis in the cis ring is required to form a GroEL-ADP-GroES complex with decreased stability. Finally, binding of ATP to the opposite trans ring of GroEL results in disassembly of the cis-ternary complex, which opens the cage and allows release of the folded protein. Proteins released in non-native form may be rapidly rebound by another GroEL complex until all of the initially bound polypeptide reaches native form. Can rescue kinetically trapped intermediates. GroEL shows ATPase activity. ATP hydrolysis moves the reaction cycle forward but is not required for substrate folding.; Also plays a role in coupling between replication of the F plasmid and cell division of the cell.; (Microbial infection) Essential for the assembly of several bacteriophages.
-
基因功能參考文獻(xiàn):
- study confirmed the altered GroEL dependence of green fluorescent protein variants with in vitro folding assays; mutations at positions predicted to be highly frustrated were found to correlate with decreased GroEL dependence; conversely, mutations at positions with low frustration were found to correlate with increased GroEL dependence PMID: 29066625
- In the case of GroEL, individualisation of monomers thus leads to individualisation of homomultimeric protein complexes, effectively providing the prerequisites for evolving an orthogonal intracellular GroEL folding machine. PMID: 24151180
- Investigated the role of the surface-associated GroEL in the binding of E. coli to macrophages. Results showed that GroEL on E. coli was recognized by LOX-1 on macrophages, leading to the phagocytosis of pathogen by macrophages. PMID: 23085345
- Nuclear magnetic resonance (NMR) observation of the uniformly (2) H,(15) N-labeled stringent 33-kDa substrate protein rhodanese in a productive complex with the uniformly (14) N-labeled 400 kDa single-ring version of the E. coli chaperonin GroEL, SR1. PMID: 21633984
- Localization of GroEL protein at possible cell division sites in penicillin treated E. coli cells suggest a possible role of the GroEL protein in cell division. PMID: 15330853
- the strong binding of N-terminal head protein sequences to GroEL has a role in promoting protein folding PMID: 15571729
- C-terminal segment of the GroEL equatorial domain has an important role in the temperature dependence of GroEL; E. coli acquired the ability to grow at low temperature through the introduction of cold-adapted chimeric or L524I mutant groEL genes. PMID: 15618620
- GroEL bound hDHFR might best be described as a dynamic ensemble of randomly structured conformers PMID: 16116078
- The natural substrate proteins for the Escherichia coli chaperonin GroEL were predicted. PMID: 16537402
- The data suggest that LDH can be successfully reactivated due to the binding of the denatured molecules to the apical domain of the opposite GroEL ring with their subsequent release into the solution without encapsulation (trans-mechanism). PMID: 16551514
- These results indicate that GroEL has at least two distinct open-conformations in the presence of nucleotide; ATP-bound prehydrolysis open-form and ADP-bound open-form, and the ATP hydrolysis in open-form destabilizes its open-conformation. PMID: 16977315
- GroEL function supports the proper folding of a majority of newly translated polypeptides PMID: 17043235
- yield of the native state in the expanded GroEL cavity is relatively small even after it remains in it for twice the spontaneous folding time PMID: 17496143
- biophysical analysis of how allosteric transitions in the chaperonin GroEL are captured by a dominant normal mode that is most robust to sequence variations PMID: 17557788
- the hydrophilic nature of 526 KNDAAD 531 residues in the flexible C-terminal region is important for proper protein folding within the central cavity of GroEL PMID: 18184659
- Forced protein unfolding is thus a central component of the multilayered stimulatory mechanism used by GroEL to drive protein folding. PMID: 18311152
- Results describe the concerted release of substrate domains from GroEL by ATP. PMID: 18556021
- GroEL provides a platform for obtaining initial glimpses of a membrane protein structure in the absence of lipids or detergents and can function as a scaffold for higher-resolution structural analysis of the protective antigen pore. PMID: 18568038
顯示更多
收起更多
-
亞細(xì)胞定位:Cytoplasm.
-
蛋白家族:Chaperonin (HSP60) family
-
數(shù)據(jù)庫鏈接:
KEGG: ecj:JW4103
STRING: 316385.ECDH10B_4336
Most popular with customers
-
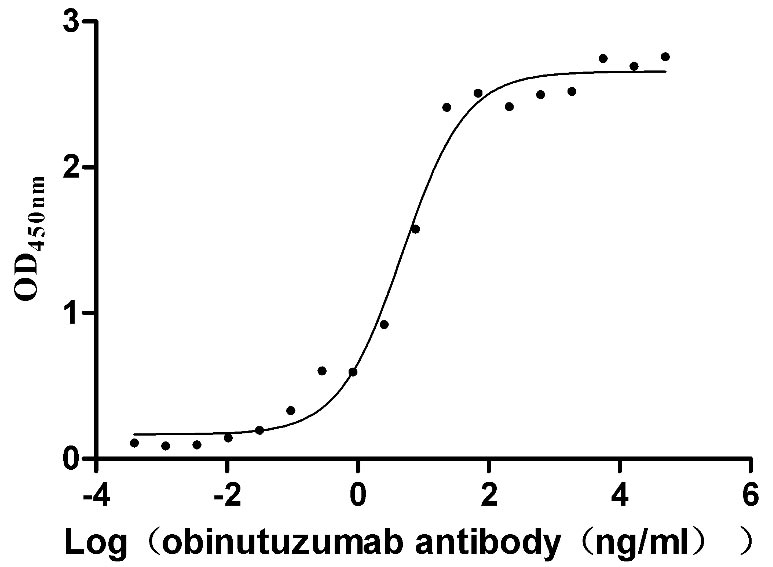
Recombinant Human B-lymphocyte antigen CD20 (MS4A1)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca mulatta Semaphorin-4D isoform 1 (SEMA4D), partial (Active)
Express system: Mammalian cell
Species: Macaca mulatta (Rhesus macaque)
-
Recombinant Macaca fascicularis Membrane spanning 4-domains A1 (MS4A1)-VLPs (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
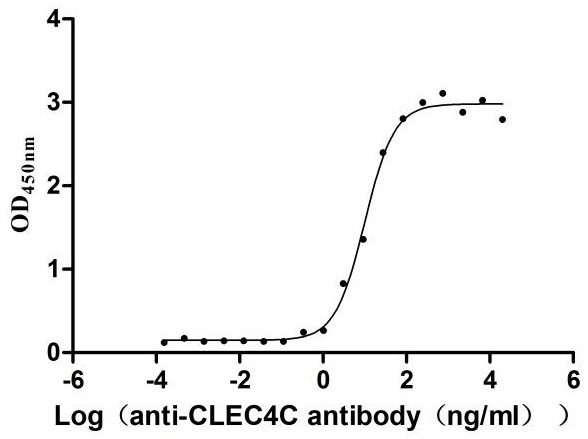
Recombinant Human C-type lectin domain family 4 member C (CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
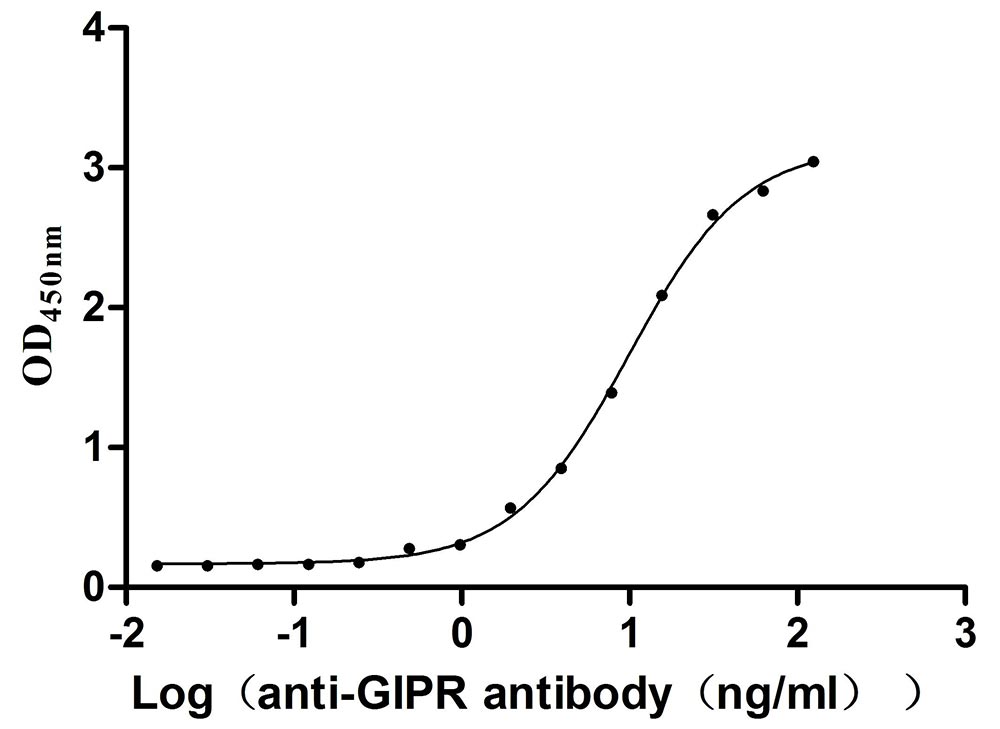
Recombinant Mouse Gastric inhibitory polypeptide receptor (Gipr), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Human Killer cell immunoglobulin-like receptor 3DL2 (KIR3DL2), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis Zinc transporter ZIP6 isoform X1(SLC39A6),partial (Active)
Express system: Baculovirus
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Macaca fascicularis Interleukin 1 receptor accessory protein(IL1RAP), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)