Recombinant Escherichia coli Chaperone protein ClpB (clpB), partial
-
中文名稱:Recombinant Escherichia coli Chaperone protein ClpB(clpB) ,partial,Yeast
-
貨號:CSB-YP352407ENV
-
規(guī)格:
-
來源:Yeast
-
其他:
-
中文名稱:Recombinant Escherichia coli Chaperone protein ClpB(clpB) ,partial,Yeast
-
貨號:CSB-EP352407ENV
-
規(guī)格:
-
來源:E.coli
-
其他:
-
中文名稱:Recombinant Escherichia coli Chaperone protein ClpB(clpB) ,partial,Yeast
-
貨號:CSB-EP352407ENV-B
-
規(guī)格:
-
來源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:Recombinant Escherichia coli Chaperone protein ClpB(clpB) ,partial,Yeast
-
貨號:CSB-BP352407ENV
-
規(guī)格:
-
來源:Baculovirus
-
其他:
-
中文名稱:Recombinant Escherichia coli Chaperone protein ClpB(clpB) ,partial,Yeast
-
貨號:CSB-MP352407ENV
-
規(guī)格:
-
來源:Mammalian cell
-
其他:
產(chǎn)品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:clpB
-
Uniprot No.:
-
別名:clpB; htpM; b2592; JW2573Chaperone protein ClpB; Heat shock protein F84.1
-
種屬:Escherichia coli (strain K12)
-
蛋白長度:Partial
-
蛋白標(biāo)簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產(chǎn)品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復(fù)溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項(xiàng):Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶點(diǎn)詳情
-
功能:Part of a stress-induced multi-chaperone system, it is involved in the recovery of the cell from heat-induced damage, in cooperation with DnaK, DnaJ and GrpE. Acts before DnaK, in the processing of protein aggregates. Protein binding stimulates the ATPase activity; ATP hydrolysis unfolds the denatured protein aggregates, which probably helps expose new hydrophobic binding sites on the surface of ClpB-bound aggregates, contributing to the solubilization and refolding of denatured protein aggregates by DnaK.
-
基因功能參考文獻(xiàn):
- The authors show here that both Escherichia coli ClpB and Saccharomyces cerevisiae Hsp104 cooperation with their cognate Hsp70 is crucial for efficient protein disaggregation and, in contrast to earlier claims, cannot be circumvented by activating M-domain mutations. PMID: 27616763
- Studied the dynamic assembly equilibrium for E. coli ClpB PMID: 26313457
- Data show that E coli can propagate the Sup35 prion, which requires disaggregase activity of the heat shock protein ClpB chaperone. PMID: 25122461
- ClpB hexamers remain associated during several ATP hydrolysis events required to translocate substrates through the protein central channel. PMID: 25558912
- Produced 7 variants of ClpB w/modified sequence of the N-terminal linker to study conformational flexibility. We conclude the linker does not merely connect the N-terminal domain, but contributes to efficiency of aggregate binding and disaggregation. PMID: 22890624
- Mutations intended to disrupt the putative ionic interactions in yeast Hsp104 and bacterial ClpB disaggregases resulted in remarkable changes of their biochemical properties PMID: 23233670
- The wt hexamer can accommodate two mutant sub units that hydrolyze ATP in only one protein ring. Four subunits seem to build the functional cooperative unit, provided that one of the protein rings contains active nucleotide binding sites. PMID: 20085762
- The authors found that ClpB95 and ClpB80 form hetero-oligomers, which are similar in size to the homo-oligomers of ClpB95 or ClpB80. PMID: 19961856
- mechanism by which ClpB couples ATP utilization to protein remodeling with and without the DnaK system PMID: 19940245
- E. coli maintains the ClpB80 to ClpB95 isoforms ratio at a nearly constant value of 0.4-0.5 under a variety of stress conditions. PMID: 16038902
- N-terminus of ClpB95 isoform interferes with its in vivo and in vitro activity PMID: 16051221
- the N-terminal domain of ClpB has an essential role in recognizing and binding strongly aggregated proteins PMID: 16076845
- These results revealed that conserved amino acids Thr7 and Ser84 both participated in maintaining the conformational integrity of the ClpB N-terminal domain. PMID: 16834329
- the conserved helix 3 of the M domain is specifically required for the DnaK-dependent shuffling of aggregated proteins, but not of soluble denatured substrates, to the pore entrance of the ClpB translocation channel. PMID: 17244532
- Certain conformational properties (in particular, beta-structures) of subunits forming these aggregates are the most important factor determining the necessity of the ClpB chaperone in the disaggregation process. PMID: 17588600
顯示更多
收起更多
-
亞細(xì)胞定位:Cytoplasm.
-
蛋白家族:ClpA/ClpB family
-
數(shù)據(jù)庫鏈接:
KEGG: ecj:JW2573
STRING: 316385.ECDH10B_2760
Most popular with customers
-
Recombinant Human Retinol-binding protein 4 (RBP4) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Paguma larvata Angiotensin-converting enzyme 2 (ACE2), partial (Active)
Express system: Mammalian cell
Species: Paguma larvata (Masked palm civet)
-
Recombinant Human Neuropilin-1 (NRP1) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Insulin growth factor-like family member 1 (IGFL1) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human E3 ubiquitin-protein ligase ZNRF3 (ZNRF3), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Complement component C1q receptor (CD93), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
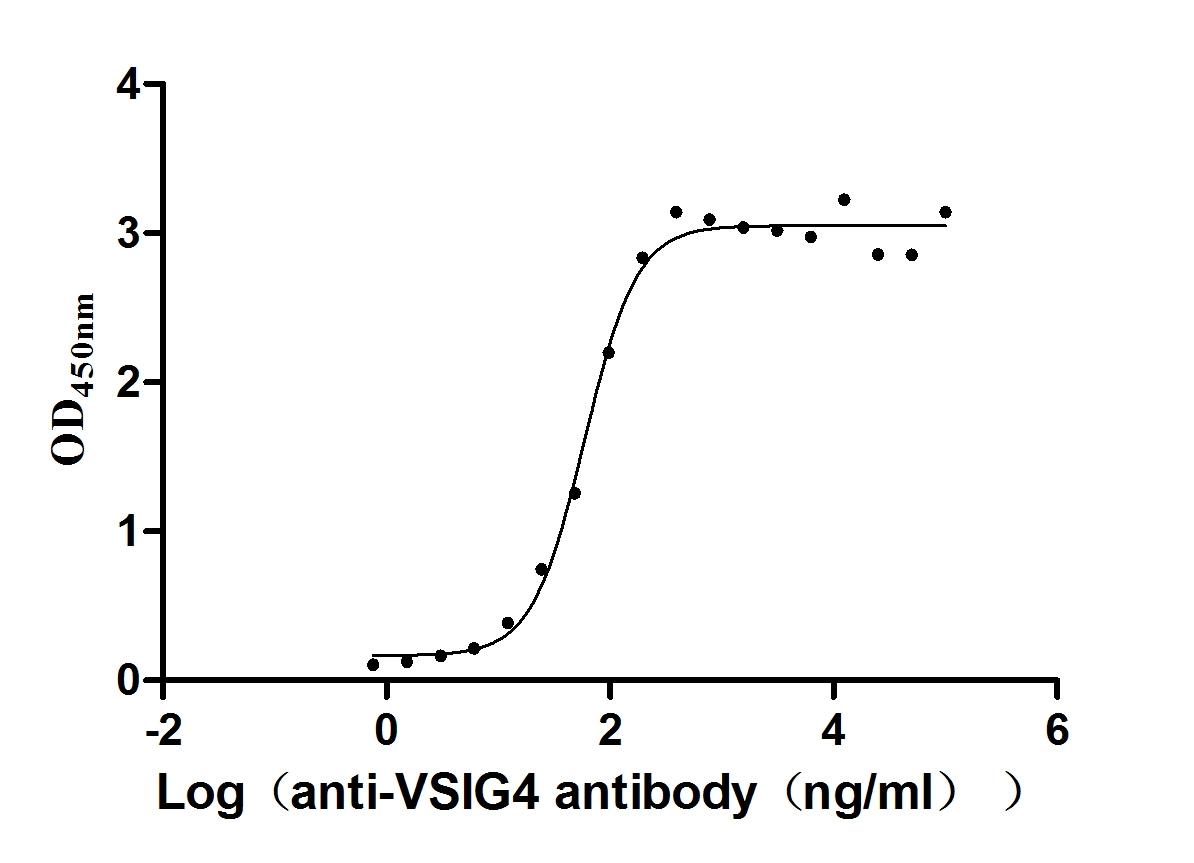
Recombinant Human V-set and immunoglobulin domain-containing protein 4 (VSIG4), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
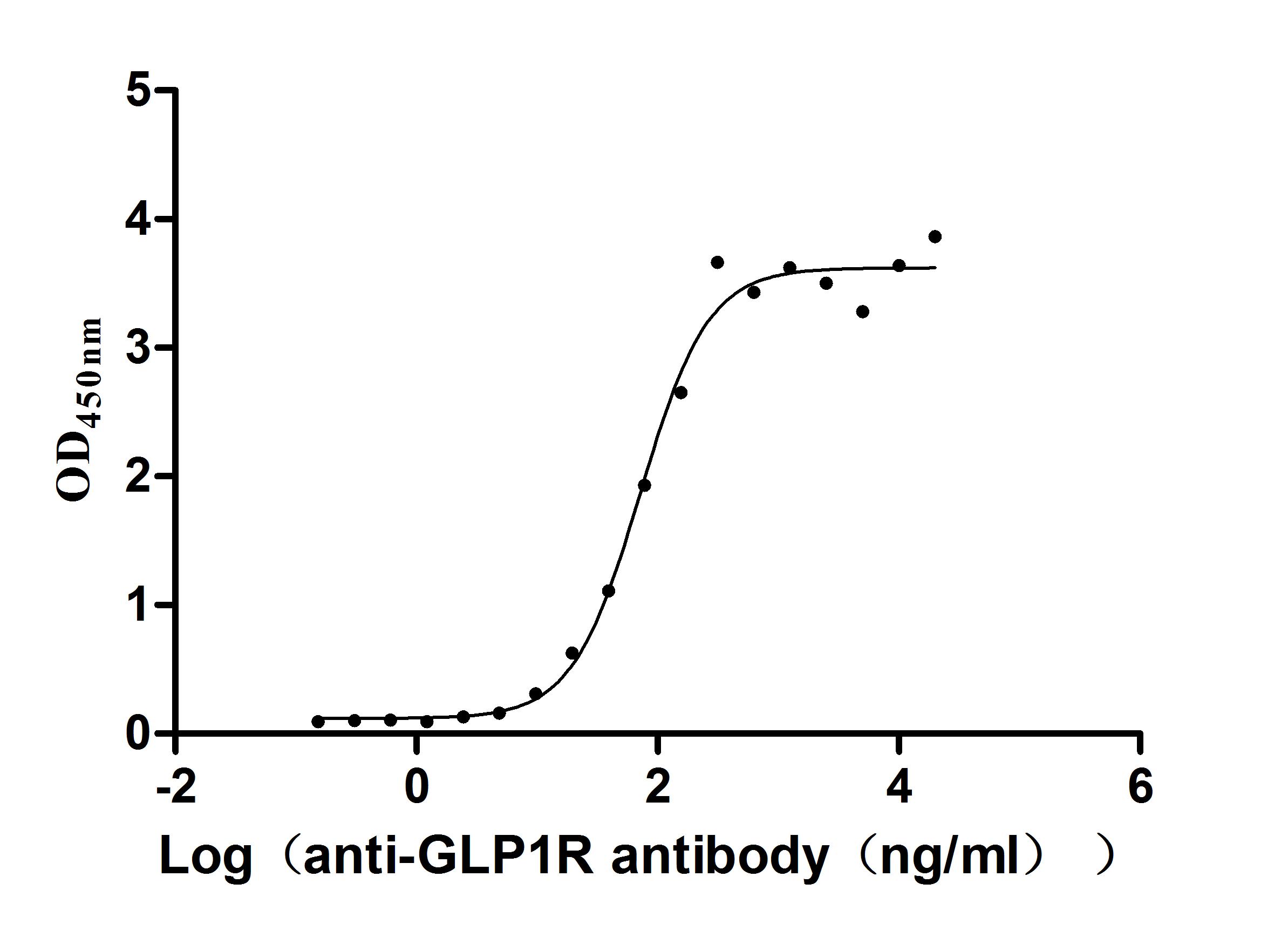
Recombinant Human Glucagon-like peptide 1 receptor (GLP1R), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)