Recombinant Escherichia coli Na (+)/H (+) antiporter nhaA, partial
-
中文名稱:大腸桿菌nhaA重組蛋白
-
貨號(hào):CSB-YP318405ENV
-
規(guī)格:
-
來(lái)源:Yeast
-
其他:
-
中文名稱:大腸桿菌nhaA重組蛋白
-
貨號(hào):CSB-EP318405ENV
-
規(guī)格:
-
來(lái)源:E.coli
-
其他:
-
中文名稱:大腸桿菌nhaA重組蛋白
-
貨號(hào):CSB-EP318405ENV-B
-
規(guī)格:
-
來(lái)源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:大腸桿菌nhaA重組蛋白
-
貨號(hào):CSB-BP318405ENV
-
規(guī)格:
-
來(lái)源:Baculovirus
-
其他:
-
中文名稱:大腸桿菌nhaA重組蛋白
-
貨號(hào):CSB-MP318405ENV
-
規(guī)格:
-
來(lái)源:Mammalian cell
-
其他:
產(chǎn)品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:nhaA
-
Uniprot No.:
-
別名:nhaA; ant; b0019; JW0018; Na(+)/H(+) antiporter NhaA; Sodium/proton antiporter NhaA
-
種屬:Escherichia coli (strain K12)
-
蛋白長(zhǎng)度:Partial
-
蛋白標(biāo)簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產(chǎn)品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復(fù)溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲(chǔ)存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項(xiàng):Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶點(diǎn)詳情
-
功能:Na(+)/H(+) antiporter that extrudes sodium in exchange for external protons. Catalyzes the exchange of 2 H(+) per Na(+). Can mediate sodium uptake when a transmembrane pH gradient is applied. Active at alkaline pH. Activity is strongly down-regulated below pH 6.5.
-
基因功能參考文獻(xiàn):
- analysis of NhaA pH-dependent activation by molecular dynamics simulation of atomic details of proton-coupled transport across membrane PMID: 27708266
- Lysine 300 has an essential role in stability but not electrogenic transport of Escherichia coli NhaA PMID: 28330875
- Data show that the Li(+) binding, H(+) release and antiporter activity were all affected to the same extent by Na(+), Li(+)/H(+) antiporter (NhaA) mutations in the Li(+) binding site (D163E, D163N, D164N, D164E). PMID: 27021484
- Data show that the two aspartic acid residues of Na+/H+ antiporter NhaA, D163 and D164, act as Na+ trap and energetic barrier for the transport of Na+. PMID: 26392528
- NhaA antiporter functions using 10 helices, and an additional 2 contribute to assembly/stability. PMID: 26417087
- As it has been previously suggested that Asp163 is one of the two residues through which proton transport occurs, these results have clear implications to the current mechanistic models of sodium-proton antiport in NhaA. PMID: 25422503
- Data indicate that binding of Li+ to purified Na(+)/H(+) antiporter NhaA is enthalpy-driven, highly specific, and pH-dependent and involves a single binding site. PMID: 22915592
- Data show that a Trp at position 136 specifically monitors a pH-induced conformational change that activates NhaA, whereas a Trp at position 339 senses a ligand-induced conformational change that does not occur until NhaA is activated at alkaline pH. PMID: 21873214
- functional and structural interactions between transmembrane domains; an active conformation of NhaA PMID: 15039449
- The molecular interactions established on Na(+) binding may represent an early step in NhaA activation. PMID: 15962009
- crystal structure of pH-downregulated NhaA, the main antiporter of Escherichia coli PMID: 15988517
- In the passive downhill uptake mode pH regulation of the carrier affects both apparent Km as well as turnover (Vmax). PMID: 16139785
- folding rates of structural segments ranged from 0.31 s(-1) to 47 s(-1), providing detailed insight into a distinct folding hierarchy of an unfolded polypeptide into the native membrane protein structure PMID: 16298390
- The protonation states of residues in the sodium proton exchanger NhaA from Escherichia coli were investigated. PMID: 16477015
- under extreme stress conditions (0.1 m LiCl or 0.7 m NaCl at pH 8.5), the dimeric native NhaA was much more efficient than the monomeric mutant in conferring extreme stress resistance. PMID: 17635927
- combinatorial approach based on molecular dynamics simulations led to formulation of a model of NhaA transport mechanism, pH regulation & cation selectivity consistent with experimental data PMID: 17690293
- Model structure of the Na+/H+ exchanger 1 in human and E. coli PMID: 17981808
- Na+/H+ antiporter genes may contribute to sodium-lithium countertransport activity and salt homeostasis in humans PMID: 18000046
- A novel structural fold creates a delicately balanced electrostatic environment in the middle of the membrane, which might be essential for ion binding and translocation. Review. PMID: 18707888
- NhaA dimers are crucial for the stability of the antiporter under extreme stress conditions. PMID: 19129192
- N-terminus verified by Edman degradation on complete protein PMID: 8381959
顯示更多
收起更多
-
亞細(xì)胞定位:Cell inner membrane; Multi-pass membrane protein.
-
蛋白家族:NhaA Na(+)/H(+) (TC 2.A.33) antiporter family
-
數(shù)據(jù)庫(kù)鏈接:
KEGG: ecj:JW0018
STRING: 316385.ECDH10B_0020
Most popular with customers
-
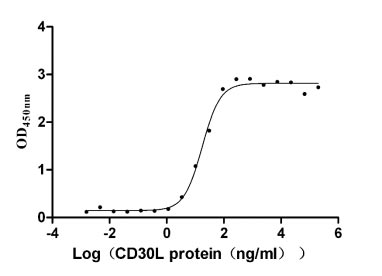
Recombinant Human Tumor necrosis factor receptor superfamily member 8 (TNFRSF8), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
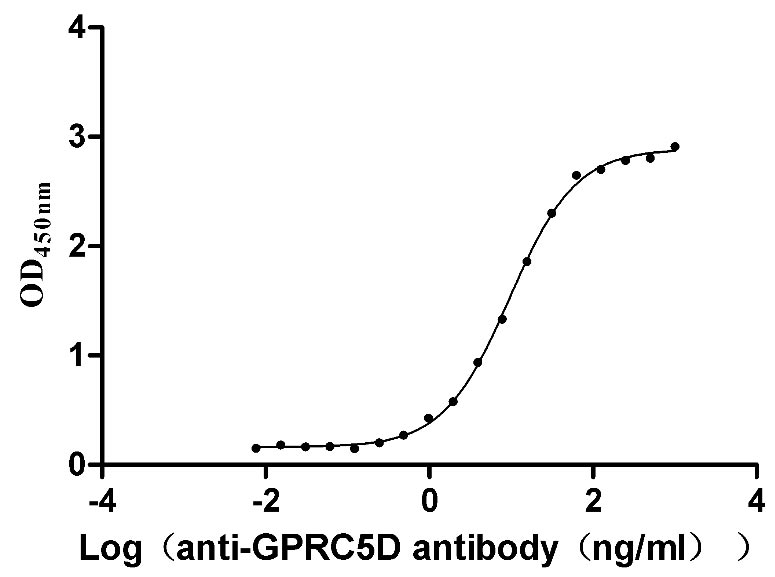
Recombinant Human G-protein coupled receptor family C group 5 member D (GPRC5D)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
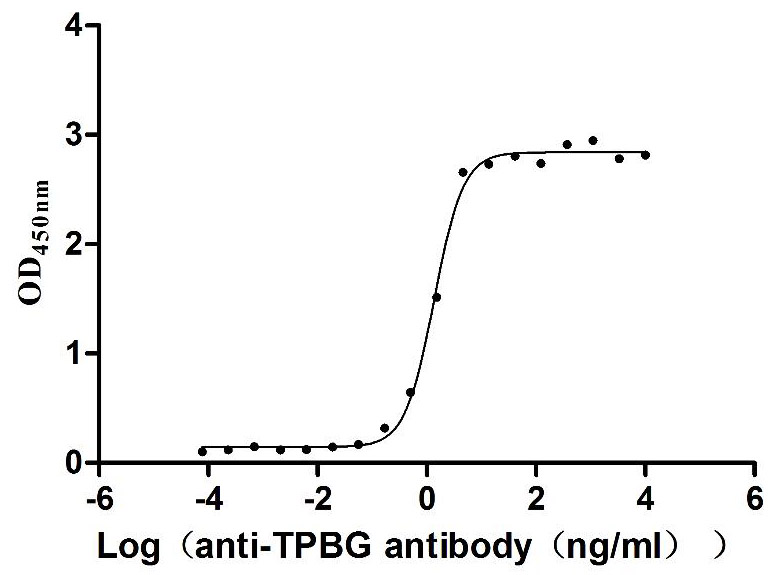
Recombinant Human Trophoblast glycoprotein (TPBG), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human C-C chemokine receptor type 6(CCR6)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Express system: Mammalian cell
Species: Homo sapiens (Human)