Recombinant Human Inactive heparanase-2 (HPSE2)
-
中文名稱:人HPSE2重組蛋白
-
貨號:CSB-YP823898HU
-
規(guī)格:
-
來源:Yeast
-
其他:
-
中文名稱:人HPSE2重組蛋白
-
貨號:CSB-EP823898HU
-
規(guī)格:
-
來源:E.coli
-
其他:
-
中文名稱:人HPSE2重組蛋白
-
貨號:CSB-EP823898HU-B
-
規(guī)格:
-
來源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:人HPSE2重組蛋白
-
貨號:CSB-BP823898HU
-
規(guī)格:
-
來源:Baculovirus
-
其他:
-
中文名稱:人HPSE2重組蛋白
-
貨號:CSB-MP823898HU
-
規(guī)格:
-
來源:Mammalian cell
-
其他:
產(chǎn)品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:HPSE2
-
Uniprot No.:
-
別名:HPSE2; HPA2; Inactive heparanase-2; Hpa2
-
種屬:Homo sapiens (Human)
-
蛋白長度:Full Length of Mature Protein
-
表達區(qū)域:42-592
-
氨基酸序列GDRRPLPVD RAAGLKEKTL ILLDVSTKNP VRTVNENFLS LQLDPSIIHD GWLDFLSSKR LVTLARGLSP AFLRFGGKRT DFLQFQNLRN PAKSRGGPGP DYYLKNYEDD IVRSDVALDK QKGCKIAQHP DVMLELQREK AAQMHLVLLK EQFSNTYSNL ILTARSLDKL YNFADCSGLH LIFALNALRR NPNNSWNSSS ALSLLKYSAS KKYNISWELG NEPNNYRTMH GRAVNGSQLG KDYIQLKSLL QPIRIYSRAS LYGPNIGRPR KNVIALLDGF MKVAGSTVDA VTWQHCYIDG RVVKVMDFLK TRLLDTLSDQ IRKIQKVVNT YTPGKKIWLE GVVTTSAGGT NNLSDSYAAG FLWLNTLGML ANQGIDVVIR HSFFDHGYNH LVDQNFNPLP DYWLSLLYKR LIGPKVLAVH VAGLQRKPRP GRVIRDKLRI YAHCTNHHNH NYVRGSITLF IINLHRSRKK IKLAGTLRDK LVHQYLLQPY GQEGLKSKSV QLNGQPLVMV DDGTLPELKP RPLRAGRTLV IPPVTMGFYV VKNVNALACR YR
-
蛋白標簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產(chǎn)品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復(fù)溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相關(guān)產(chǎn)品
靶點詳情
-
功能:Binds heparin and heparan sulfate with high affinity, but lacks heparanase activity. Inhibits HPSE, possibly by competing for its substrates (in vitro).
-
基因功能參考文獻:
- The results of this study the Heparanase 2 appeared overexpressed at different stages of Alzheimer disease. PMID: 28387673
- our results suggest that heparanase 2 functions as a tumor suppressor in bladder cancer PMID: 26968815
- we provide evidence that Hpa2 overexpression in head and neck cancer cells markedly reduces tumor growth PMID: 27013193
- The most common HPA genotypes among Saudis were HPA-1 a + b- (75%), HPA-2 a + b- (62%), HPA-3 a + b- (51.5%), HPA-4 a + b- (99%), HPA-5 a + b- (76.5%), HPA-6 a + b- (100%) and HPA-15 a + b + (50%). The prevalent allele among the HPA systems was (a), except in the HPA-15 system where the (b) allele was found in 52% of the subjects. PMID: 27019315
- Our findings identified these 2 genes as a novel breast cancer biomarker gene set, which may facilitate the diagnosis and treatment in breast cancer clinical therapies. PMID: 26084486
- HPSE2 mutations were found in one Urofacial syndrome family but not detected in patients with non-neurogenic neurogenic bladder and severe lower urinary tract dysfunction PMID: 25924634
- Heparanase 2 is more intensely expressed in the glandular tissue of cancer than in nonneoplastic endometrium; the HPSE2 expression in the stromal tissue is higher in the nonneoplastic controls compared with cancer mainly in the secretory endometrium. PMID: 25423319
- autonomic neural protein implicated in bladder emptying PMID: 25145936
- High expression of heparanase-2 is associated significantly with gastric tumor growth and differentiation PMID: 24139593
- Data indicate that the overexpression of HPSE1 and HPSE2 in the intervertebral degenerated discs suggests a role for these factors in mediating extracellular matrix remodeling in degenerative discs during disease development. PMID: 23370684
- HPSE2 c.631T>C (p.Y211H) is a novel benign SNP and c.1628A>T (p.N543I) is the disease-causing mutation in urofacial syndrome. PMID: 21332471
- A large region of marker homozygosity was observed at 10q24, consistent with known autosomal recessive inheritance, family consanguinity and previous genetic mapping in other families with Ochoa syndrome. PMID: 21450525
- Studies indicate that cathepsin L as the heparanase activating protease. PMID: 21308479
- These results indicate a regulatory effect of heparanase on TFPI and TFPI-2 in trophoblasts, suggesting a potential involvement of heparanase in early miscarriages. PMID: 20031192
- We now report evidence that Heparanse 2 (HPSE2) is the culprit gene for the syndrome. Mutations with a loss of function in the Heparanase 2 (HPSE2) gene PMID: 20560209
- Homozygous exonic deletions, nonsense mutations, and frameshift mutations in five further unrelated families confirmed HPSE2 as the causative gene for UFS PMID: 20560210
- Results demonstrated that the effectors from heparanase peptide-immunized mice could effectively lyse various tumor cells that were heparanase positive and HLA-A*0201 matched. PMID: 20182872
- Heparanase 2 is involved in neoplastic proliferation, but it was not exclusively associated with the malignant process. PMID: 19955924
- Heparanase may facilitate invasion and metastasis of gastric carcinoma cells. PMID: 15475937
- Study demonstrating that increased heparanase expression in prostate cancer tissues is due to promoter hypomethylation and up-regulation of transcription factor EGR1. PMID: 15709168
- Heparanase expression seems to be involved in the invasiveness and aggressiveness of head and neck squamous cell carcinomas. PMID: 15837740
- Heparanase expression is inversely correlated with survival of nasopharyngeal carcinoma (NPC) patients, indicating heparanase is a reliable prognostic factor, and further supports that heparanase is a valid target for the development of anti-cancer drugs. PMID: 16879396
- HPSE plays a role in extracellular matrix remodeling and in increasing heparin-binding growth factor release during embryo implantation. PMID: 17989358
- Data suggest that loss of modified heparan sulphate in the GBM is mediated by an increased heparanase presence and may play a role in the pathogenesis of diabetes-induced proteinuria. PMID: 18058084
顯示更多
收起更多
-
相關(guān)疾病:Urofacial syndrome 1 (UFS1)
-
亞細胞定位:Secreted, extracellular space, extracellular matrix.
-
蛋白家族:Glycosyl hydrolase 79 family
-
組織特異性:Widely expressed, with the highest expression in brain, mammary gland, prostate, small intestine, testis and uterus. In the central nervous system, expressed in the spinal chord, caudate nucleus, thalamus, substantia nigra, medulla oblongata, putamen and
-
數(shù)據(jù)庫鏈接:
Most popular with customers
-
Recombinant Human C-X-C chemokine receptor type 4 (CXCR4)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
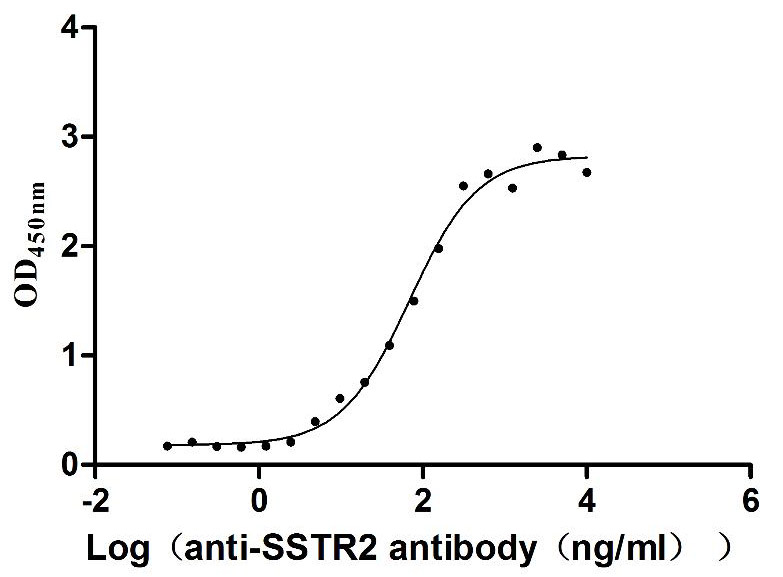
Recombinant Human Somatostatin receptor type 2 (SSTR2)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Complement component C1q receptor (CD93), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
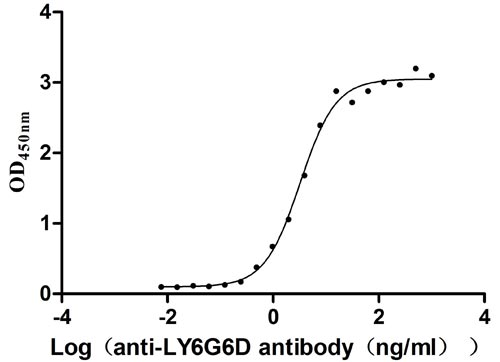
Recombinant Human Lymphocyte antigen 6 complex locus protein G6d (LY6G6D) (Active)
Express system: Yeast
Species: Homo sapiens (Human)
-
Recombinant Mouse Cell adhesion molecule 1 (Cadm1), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Human Gastric inhibitory polypeptide receptor(GIPR),partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Kidney-associated antigen 1(KAAG1) (Active)
Express system: E.coli
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis Zinc transporter ZIP6 isoform X1(SLC39A6),partial (Active)
Express system: Baculovirus
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)


-AC1.jpg)