Recombinant Influenza A virus Hemagglutinin (HA), partial
-
中文名稱(chēng):Recombinant Influenza A virus Hemagglutinin(HA) ,partial,Yeast
-
貨號(hào):CSB-YP365947IFZ
-
規(guī)格:
-
來(lái)源:Yeast
-
其他:
-
中文名稱(chēng):Recombinant Influenza A virus Hemagglutinin(HA) ,partial,Yeast
-
貨號(hào):CSB-EP365947IFZ
-
規(guī)格:
-
來(lái)源:E.coli
-
其他:
-
中文名稱(chēng):Recombinant Influenza A virus Hemagglutinin(HA) ,partial,Yeast
-
貨號(hào):CSB-EP365947IFZ-B
-
規(guī)格:
-
來(lái)源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱(chēng):Recombinant Influenza A virus Hemagglutinin(HA) ,partial,Yeast
-
貨號(hào):CSB-BP365947IFZ
-
規(guī)格:
-
來(lái)源:Baculovirus
-
其他:
-
中文名稱(chēng):Recombinant Influenza A virus Hemagglutinin(HA) ,partial,Yeast
-
貨號(hào):CSB-MP365947IFZ
-
規(guī)格:
-
來(lái)源:Mammalian cell
-
其他:
產(chǎn)品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:HA
-
Uniprot No.:
-
別名:HA; Hemagglutinin [Cleaved into: Hemagglutinin HA1 chain; Hemagglutinin HA2 chain]
-
種屬:Influenza A virus (strain A/Puerto Rico/8/1934 H1N1)
-
蛋白長(zhǎng)度:Partial
-
蛋白標(biāo)簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產(chǎn)品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復(fù)溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲(chǔ)存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項(xiàng):Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶點(diǎn)詳情
-
功能:Binds to sialic acid-containing receptors on the cell surface, bringing about the attachment of the virus particle to the cell. This attachment induces virion internalization either through clathrin-dependent endocytosis or through clathrin- and caveolin-independent pathway. Plays a major role in the determination of host range restriction and virulence. Class I viral fusion protein. Responsible for penetration of the virus into the cell cytoplasm by mediating the fusion of the membrane of the endocytosed virus particle with the endosomal membrane. Low pH in endosomes induces an irreversible conformational change in HA2, releasing the fusion hydrophobic peptide. Several trimers are required to form a competent fusion pore.
-
基因功能參考文獻(xiàn):
- Exploration of binding and inhibition mechanism of a small molecule inhibitor of influenza virus H1N1 hemagglutinin by molecular dynamics simulation. PMID: 28630402
- cellular kinase, casein kinase 1alpha (CK1alpha), is crucial for IAV HA-induced degradation of both IFNGR1 and IFNAR1. PMID: 29343571
- MAb, MA-H generated against the seasonal A/Solomon Islands/03/2006 (H1N1) rHA binds within the head domain and bound the seasonal H1N1 (A/Solomon Islands/03/2006 and A/New Caledonia/20/1990) rHAs with high affinity (KD; 0.72-8.23 nM). MA-H showed high HI (2.50 mug/mL) and in vitro neutralization (IC50 = 2.61 mug/mL) activity against the A/Solomon Islands/03/2006 virus PMID: 27463230
- FLUAV strains can counteract tetherin via their HA and NA proteins identifies these proteins as novel tetherin antagonists and indicates that HA/NA-dependent inactivation of innate defenses may contribute to the efficient spread of pandemic FLUAV. PMID: 26109730
- responsible for the reduced threshold pH for fusion. The higher structural and acid stability of the HA trimer caused by the E47K change also conferred higher viral thermal stability and infectivity PMID: 24391498
- the Sa antigenic site is immunodominant in pdm09 HA, whereas the N203D mutation (Sb site), present in three different Bris07 escape mutants, appears as the immunodominant epitope in that strain PMID: 25078301
- These results not only reinforce the association between D222G substitution and influenza A(H1N1)pdm09-associated morbidity and mortality PMID: 24667815
- findings suggest that recent A(H1N1)pdm09 viruses are now more permissive to the acquisition of H275Y than earlier A(H1N1)pdm09 viruses, increasing the risk that OR A(H1N1)pdm09 will emerge and spread worldwide. PMID: 24699865
- Coexpression experiments revealed that apical targeting of HA and NA was accelerated by their coexpression. PMID: 24965459
- Stem immunogens designed from unmatched, highly drifted influenza strains conferred robust protection against a lethal heterologous A/Puerto Rico/8/34 virus challenge in vivo. PMID: 24927560
- This model implies that the ability to rapidly acquire mutations is an inherent aspect of influenza HA and nonstructural protein 1 proteins. PMID: 24277853
- Characterization of the major mutations of the heammaglutinin gene found in Tunisian pandemic strains. PMID: 23679923
- S186P and S188T amino acid substitutions increase the receptor-binding avidity of HA. PMID: 24109242
- Q223R mutation in the HA changes the HA binding preference from the human-type receptor, alpha2,6-linked sialic acid, to the avian-type receptor, alpha2,3-linked sialic acid. PMID: 24020758
- Polymorphism in H1 HA at position 147 modulates viral fitness by buffering the constraints caused by N-linked glycans. PMID: 23637398
- Substitutions T200A and E227A in the hemagglutinin influenza HIN1 virus increase lethality but decrease transmission. PMID: 23536663
- HA and the viral RNA polymerase complex enhance viral pathogenicity, but only HA induces aberrant host responses in mice. PMID: 23449804
- Cleavage activation of HA by plasmin/plasminogen was independent of the viral NA. PMID: 23449787
- The binding free energy calculation indicates that the D222G mutated hemagglutinin has a much stronger binding affinity with the studied alpha2,3-linked glycan than the wild type. PMID: 22581100
- amino acid 271A of PB2 plays a key role in virus acquisition of the mutation at position 226 of HA that confers human receptor recognition PMID: 22740390
- The human 2009 H1N1 pandemic virus exhibited low HA avidity for glycan receptors and weak NA enzymatic activity. PMID: 22718832
- The G222 mutation of HA was the most pathogenic in ferrets. PMID: 22575875
- Evidence that, despite the highly conserved genetic nature of H1N1pdm09, a number of mutations in antigenically critical epitopes occurred in the HA1 domain of hemagglutinin gene of pandemic influenza H1N1pdm09 viruses. PMID: 22246823
- Asp190 and Asp225 of HA play critical roles in glycan recognition through hydrogen bonds with GlcNAc-3 and Gal-2. PMID: 22072785
- Missense mutation at residue 82, 141, or 189 of the HA protein promotes virus replication in the presence of the neuraminidase H274Y mutation. PMID: 22013054
- Recombinant HA1 proteins were shown to form functional oligomers of trimers, similar to virus derived HA, and elicited high titer of neutralizing antibodies in rabbits and sheep. PMID: 21704111
- Results indicate that residue 222 of the hemagglutinin protein may affect the positioning of the conserved Q223 residue, hence modulating flexibility of the binding pocket and steric hindrance during receptor binding. PMID: 21727184
- Data show that both HA-S220T and NA-N248D are major non-synonymous mutations that clearly discriminate the 2009 pdm influenza viruses identified in the very early phase in Japan. PMID: 21572517
- The HA1 gene of novel influenza virus might originate from the early swine H1N1 influenza virus from North America. PMID: 20117993
- Both HA and NA genes are under the strongest positive selection. PMID: 21507270
- T220 and E/G239 mutations of HA were under positive selection during the spreading of A/H1N1/09 virus worldwide. PMID: 20809098
- A cluster of D222E viruses (of influenza A(H1N1)) among school children confirms reported human-to-human transmission of viruses mutated at amino acid position 222. PMID: 21087581
- D222G hemagglutinin mutant altered receptor specificity and cell tropism. PMID: 20826688
- Substitution D222G in the viral hemagglutinin increased receptor binding. PMID: 20844044
- The Q223R mutation in the HA receptor bining site greatly increases infectivity without affecting antigenicity. PMID: 20869738
- the influenza hemagglutinin fusion domain adopts a tight helical hairpin arrangement at the lipid:water interface PMID: 20534508
顯示更多
收起更多
-
亞細(xì)胞定位:Virion membrane; Single-pass type I membrane protein. Host apical cell membrane; Single-pass type I membrane protein.
-
蛋白家族:Influenza viruses hemagglutinin family
-
數(shù)據(jù)庫(kù)鏈接:
KEGG: vg:956529
Most popular with customers
-
Recombinant Human Delta-like protein 3 (DLL3), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis Delta-like protein 3 (DLL3), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
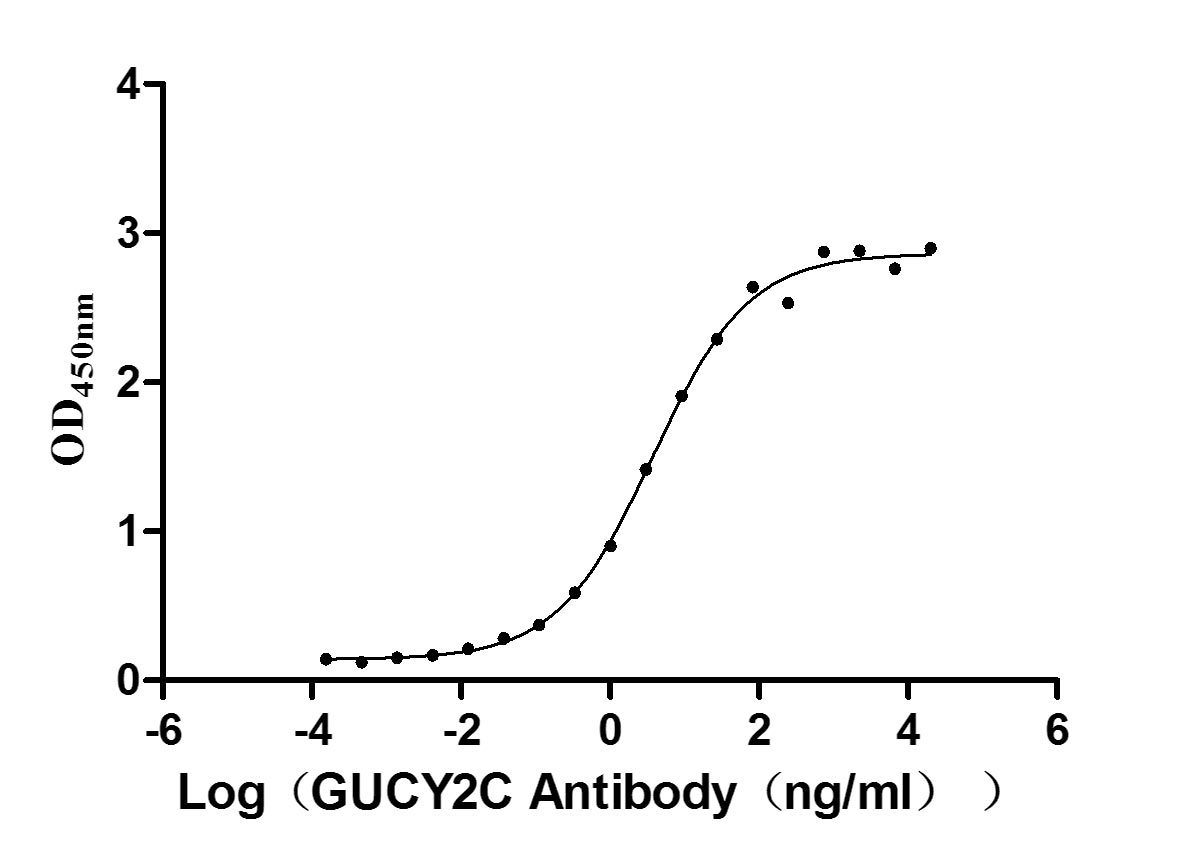
Recombinant Human Heat-stable enterotoxin receptor (GUCY2C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Dog B-lymphocyte antigen CD20 (MS4A1)-VLPs (Active)
Express system: Mammalian cell
Species: Canis lupus familiaris (Dog) (Canis familiaris)
-
Recombinant Human Microtubule-associated protein tau (MAPT) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse Cadherin-6(Cdh6),partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)





-AC1.jpg)