Recombinant Mouse NAD-dependent protein deacetylase sirtuin-1 (Sirt1)
-
中文名稱:小鼠Sirt1重組蛋白
-
貨號(hào):CSB-YP846058MO
-
規(guī)格:
-
來源:Yeast
-
其他:
-
中文名稱:小鼠Sirt1重組蛋白
-
貨號(hào):CSB-EP846058MO
-
規(guī)格:
-
來源:E.coli
-
其他:
-
中文名稱:小鼠Sirt1重組蛋白
-
貨號(hào):CSB-EP846058MO-B
-
規(guī)格:
-
來源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:小鼠Sirt1重組蛋白
-
貨號(hào):CSB-BP846058MO
-
規(guī)格:
-
來源:Baculovirus
-
其他:
-
中文名稱:小鼠Sirt1重組蛋白
-
貨號(hào):CSB-MP846058MO
-
規(guī)格:
-
來源:Mammalian cell
-
其他:
產(chǎn)品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
別名:Sirt1; Sir2l1; NAD-dependent protein deacetylase sirtuin-1; EC 2.3.1.286; NAD-dependent protein deacylase sirtuin-1; EC 2.3.1.-; Regulatory protein SIR2 homolog 1; SIR2-like protein 1; SIR2alpha; Sir2; mSIR2a) [Cleaved into: SirtT1 75 kDa fragment; 75SirT1)]
-
種屬:Mus musculus (Mouse)
-
蛋白長度:Full Length of Mature Protein
-
表達(dá)區(qū)域:2-737
-
氨基酸序列ADEVALALQ AAGSPSAAAA MEAASQPADE PLRKRPRRDG PGLGRSPGEP SAAVAPAAAG CEAASAAAPA ALWREAAGAA ASAEREAPAT AVAGDGDNGS GLRREPRAAD DFDDDEGEEE DEAAAAAAAA AIGYRDNLLL TDGLLTNGFH SCESDDDDRT SHASSSDWTP RPRIGPYTFV QQHLMIGTDP RTILKDLLPE TIPPPELDDM TLWQIVINIL SEPPKRKKRK DINTIEDAVK LLQECKKIIV LTGAGVSVSC GIPDFRSRDG IYARLAVDFP DLPDPQAMFD IEYFRKDPRP FFKFAKEIYP GQFQPSLCHK FIALSDKEGK LLRNYTQNID TLEQVAGIQR ILQCHGSFAT ASCLICKYKV DCEAVRGDIF NQVVPRCPRC PADEPLAIMK PEIVFFGENL PEQFHRAMKY DKDEVDLLIV IGSSLKVRPV ALIPSSIPHE VPQILINREP LPHLHFDVEL LGDCDVIINE LCHRLGGEYA KLCCNPVKLS EITEKPPRPQ KELVHLSELP PTPLHISEDS SSPERTVPQD SSVIATLVDQ ATNNNVNDLE VSESSCVEEK PQEVQTSRNV ENINVENPDF KAVGSSTADK NERTSVAETV RKCWPNRLAK EQISKRLEGN QYLFVPPNRY IFHGAEVYSD SEDDVLSSSS CGSNSDSGTC QSPSLEEPLE DESEIEEFYN GLEDDTERPE CAGGSGFGAD GGDQEVVNEA IATRQELTDV NYPSDKS
-
蛋白標(biāo)簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產(chǎn)品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復(fù)溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲(chǔ)存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項(xiàng):Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相關(guān)產(chǎn)品
靶點(diǎn)詳情
-
功能:NAD-dependent protein deacetylase that links transcriptional regulation directly to intracellular energetics and participates in the coordination of several separated cellular functions such as cell cycle, response to DNA damage, metabolism, apoptosis and autophagy. Can modulate chromatin function through deacetylation of histones and can promote alterations in the methylation of histones and DNA, leading to transcriptional repression. Deacetylates a broad range of transcription factors and coregulators, thereby regulating target gene expression positively and negatively. Serves as a sensor of the cytosolic ratio of NAD(+)/NADH which is altered by glucose deprivation and metabolic changes associated with caloric restriction. Is essential in skeletal muscle cell differentiation and in response to low nutrients mediates the inhibitory effect on skeletal myoblast differentiation which also involves 5'-AMP-activated protein kinase (AMPK) and nicotinamide phosphoribosyltransferase (NAMPT). Component of the eNoSC (energy-dependent nucleolar silencing) complex, a complex that mediates silencing of rDNA in response to intracellular energy status and acts by recruiting histone-modifying enzymes. The eNoSC complex is able to sense the energy status of cell: upon glucose starvation, elevation of NAD(+)/NADP(+) ratio activates SIRT1, leading to histone H3 deacetylation followed by dimethylation of H3 at 'Lys-9' (H3K9me2) by SUV39H1 and the formation of silent chromatin in the rDNA locus. Deacetylates 'Lys-266' of SUV39H1, leading to its activation. Inhibits skeletal muscle differentiation by deacetylating PCAF and MYOD1. Deacetylates H2A and 'Lys-26' of H1-4. Deacetylates 'Lys-16' of histone H4 (in vitro). Involved in NR0B2/SHP corepression function through chromatin remodeling: Recruited to LRH1 target gene promoters by NR0B2/SHP thereby stimulating histone H3 and H4 deacetylation leading to transcriptional repression. Proposed to contribute to genomic integrity via positive regulation of telomere length; however, reports on localization to pericentromeric heterochromatin are conflicting. Proposed to play a role in constitutive heterochromatin (CH) formation and/or maintenance through regulation of the available pool of nuclear SUV39H1. Upon oxidative/metabolic stress decreases SUV39H1 degradation by inhibiting SUV39H1 polyubiquitination by MDM2. This increase in SUV39H1 levels enhances SUV39H1 turnover in CH, which in turn seems to accelerate renewal of the heterochromatin which correlates with greater genomic integrity during stress response. Deacetylates 'Lys-382' of p53/TP53 and impairs its ability to induce transcription-dependent proapoptotic program and modulate cell senescence. Deacetylates TAF1B and thereby represses rDNA transcription by the RNA polymerase I. Deacetylates MYC, promotes the association of MYC with MAX and decreases MYC stability leading to compromised transformational capability. Deacetylates FOXO3 in response to oxidative stress thereby increasing its ability to induce cell cycle arrest and resistance to oxidative stress but inhibiting FOXO3-mediated induction of apoptosis transcriptional activity; also leading to FOXO3 ubiquitination and protesomal degradation. Appears to have a similar effect on MLLT7/FOXO4 in regulation of transcriptional activity and apoptosis. Deacetylates DNMT1; thereby impairs DNMT1 methyltransferase-independent transcription repressor activity, modulates DNMT1 cell cycle regulatory function and DNMT1-mediated gene silencing. Deacetylates RELA/NF-kappa-B p65 thereby inhibiting its transactivating potential and augments apoptosis in response to TNF-alpha. Deacetylates HIF1A, KAT5/TIP60, RB1 and HIC1. Deacetylates FOXO1, which increases its DNA binding ability and enhances its transcriptional activity leading to increased gluconeogenesis in liver. Inhibits E2F1 transcriptional activity and apoptotic function, possibly by deacetylation. Involved in HES1- and HEY2-mediated transcriptional repression. In cooperation with MYCN seems to be involved in transcriptional repression of DUSP6/MAPK3 leading to MYCN stabilization by phosphorylation at 'Ser-62'. Deacetylates MEF2D. Required for antagonist-mediated transcription suppression of AR-dependent genes which may be linked to local deacetylation of histone H3. Represses HNF1A-mediated transcription. Required for the repression of ESRRG by CREBZF. Deacetylates NR1H3 and NR1H2 and deacetylation of NR1H3 at 'Lys-434' positively regulates transcription of NR1H3:RXR target genes, promotes NR1H3 proteosomal degradation and results in cholesterol efflux; a promoter clearing mechanism after reach round of transcription is proposed. Involved in lipid metabolism. Implicated in regulation of adipogenesis and fat mobilization in white adipocytes by repression of PPARG which probably involves association with NCOR1 and SMRT/NCOR2. Deacetylates p300/EP300 and PRMT1. Deacetylates ACSS2 leading to its activation, and HMGCS1 deacetylation. Involved in liver and muscle metabolism. Through deacetylation and activation of PPARGC1A is required to activate fatty acid oxidation in skeletal muscle under low-glucose conditions and is involved in glucose homeostasis. Involved in regulation of PPARA and fatty acid beta-oxidation in liver. Involved in positive regulation of insulin secretion in pancreatic beta cells in response to glucose; the function seems to imply transcriptional repression of UCP2. Proposed to deacetylate IRS2 thereby facilitating its insulin-induced tyrosine phosphorylation. Deacetylates SREBF1 isoform SREBP-1C thereby decreasing its stability and transactivation in lipogenic gene expression. Involved in DNA damage response by repressing genes which are involved in DNA repair, such as XPC and TP73, deacetylating XRCC6/Ku70, and facilitating recruitment of additional factors to sites of damaged DNA, such as SIRT1-deacetylated NBN can recruit ATM to initiate DNA repair and SIRT1-deacetylated XPA interacts with RPA2. Also involved in DNA repair of DNA double-strand breaks by homologous recombination and specifically single-strand annealing independently of XRCC6/Ku70 and NBN. Transcriptional suppression of XPC probably involves an E2F4:RBL2 suppressor complex and protein kinase B (AKT) signaling. Transcriptional suppression of TP73 probably involves E2F4 and PCAF. Deacetylates WRN thereby regulating its helicase and exonuclease activities and regulates WRN nuclear translocation in response to DNA damage. Deacetylates APEX1 at 'Lys-6' and 'Lys-7' and stimulates cellular AP endonuclease activity by promoting the association of APEX1 to XRCC1. Increases p53/TP53-mediated transcription-independent apoptosis by blocking nuclear translocation of cytoplasmic p53/TP53 and probably redirecting it to mitochondria. Deacetylates XRCC6/Ku70 at 'Lys-537' and 'Lys-540' causing it to sequester BAX away from mitochondria thereby inhibiting stress-induced apoptosis. Is involved in autophagy, presumably by deacetylating ATG5, ATG7 and MAP1LC3B/ATG8. Deacetylates AKT1 which leads to enhanced binding of AKT1 and PDK1 to PIP3 and promotes their activation. Proposed to play role in regulation of STK11/LBK1-dependent AMPK signaling pathways implicated in cellular senescence which seems to involve the regulation of the acetylation status of STK11/LBK1. Can deacetylate STK11/LBK1 and thereby increase its activity, cytoplasmic localization and association with STRAD; however, the relevance of such activity in normal cells is unclear. In endothelial cells is shown to inhibit STK11/LBK1 activity and to promote its degradation. Deacetylates SMAD7 at 'Lys-64' and 'Lys-70' thereby promoting its degradation. Deacetylates CIITA and augments its MHC class II transactivation and contributes to its stability. Deacetylates MECOM/EVI1. Deacetylates PML at 'Lys-487' and this deacetylation promotes PML control of PER2 nuclear localization. During the neurogenic transition, represses selective NOTCH1-target genes through histone deacetylation in a BCL6-dependent manner and leading to neuronal differentiation. Regulates the circadian expression of several core clock genes, including ARNTL/BMAL1, RORC, PER2 and CRY1 and plays a critical role in maintaining a controlled rhythmicity in histone acetylation, thereby contributing to circadian chromatin remodeling. Deacetylates ARNTL/BMAL1 and histones at the circadian gene promoters in order to facilitate repression by inhibitory components of the circadian oscillator. Deacetylates PER2, facilitating its ubiquitination and degradation by the proteosome. Protects cardiomyocytes against palmitate-induced apoptosis. Deacetylates XBP1 isoform 2; deacetylation decreases protein stability of XBP1 isoform 2 and inhibits its transcriptional activity. Deacetylates PCK1 and directs its activity toward phosphoenolpyruvate production promoting gluconeogenesis. Involved in the CCAR2-mediated regulation of PCK1 and NR1D1. Deacetylates CTNB1 at 'Lys-49'. In POMC (pro-opiomelanocortin) neurons, required for leptin-induced activation of PI3K signaling. In addition to protein deacetylase activity, also acts as protein-lysine deacylase: acts as a protein depropionylase by mediating depropionylation of Osterix (SP7). Deacetylates SOX9; promoting SOX9 nuclear localization and transactivation activity. Involved in the regulation of centrosome duplication. Deacetylates CENATAC in G1 phase, allowing for SASS6 accumulation on the centrosome and subsequent procentriole assembly. {ECO:0000250|UniProtKB:Q96EB6, ECO:0000269|PubMed:11250901, ECO:0000269|PubMed:11672522, ECO:0000269|PubMed:12651913, ECO:0000269|PubMed:12887892, ECO:0000269|PubMed:12960381, ECO:0000269|PubMed:15175761, ECO:0000269|PubMed:15220471, ECO:0000269|PubMed:15632193, ECO:0000269|PubMed:15744310, ECO:0000269|PubMed:15788402, ECO:0000269|PubMed:16098828, ECO:0000269|PubMed:16366736, ECO:0000269|PubMed:16790548, ECO:0000269|PubMed:16892051, ECO:0000269|PubMed:17098745, ECO:0000269|PubMed:17347648, ECO:0000269|PubMed:17620057, ECO:0000269|PubMed:17901049, ECO:0000269|PubMed:17936707, ECO:0000269|PubMed:18004385, ECO:0000269|PubMed:18296641, ECO:0000269|PubMed:18371449, ECO:0000269|PubMed:18477450, ECO:0000269|PubMed:18662546, ECO:0000269|PubMed:18662547, ECO:0000269|PubMed:18687677, ECO:0000269|PubMed:19299583, ECO:0000269|PubMed:19356714, ECO:0000269|PubMed:20167603, ECO:0000269|PubMed:20620997, ECO:0000269|PubMed:20817729, ECO:0000269|PubMed:21176092, ECO:0000269|PubMed:21187328, ECO:0000269|PubMed:21189328, ECO:0000269|PubMed:21622680, ECO:0000269|PubMed:23160044, ECO:0000269|PubMed:26910618, ECO:0000269|PubMed:28883095, ECO:0000269|PubMed:30026585, ECO:0000269|PubMed:30193097}.; [Isoform 2]: Deacetylates 'Lys-382' of p53/TP53, however with lower activity than isoform 1. In combination, the two isoforms exert an additive effect. Isoform 2 regulates p53/TP53 expression and cellular stress response and is in turn repressed by p53/TP53 presenting a SIRT1 isoform-dependent auto-regulatory loop. {ECO:0000250|UniProtKB:Q96EB6}.; [SirtT1 75 kDa fragment]: Catalytically inactive 75SirT1 may be involved in regulation of apoptosis. May be involved in protecting chondrocytes from apoptotic death by associating with cytochrome C and interfering with apoptosome assembly. {ECO:0000250|UniProtKB:Q96EB6}.
-
基因功能參考文獻(xiàn):
- Study found little evidence that Sirt1 promotes the development of food anticipatory activity (FAA) and no evidence for the involvement of Sirt1 in the maintenance of FAA; nor much effect on body weight homeostasis during 60% timed calorie-restricted (CR) feeding over a short-term CR experiment. PMID: 29940007
- Authors report here that SIRT1 levels were decreased in the liver in different mouse models and in cultured HSCs undergoing activation. PMID: 28919365
- Authors concluded that miR-29b is an important regulator in the PDGF-BB-mediated VSMC phenotypic transition by targeting SIRT1. PMID: 30231015
- In the absence of Sirt1, both embryos and placentas were small, with placentas showing abnormalities in both the labyrinthine layer and junctional zone. Sirt1-null trophoblastic stem cells showed blunted differentiation, and appeared to be suspended in an Epcam(high) trophoblast progenitor state. PMID: 29405961
- the Sirt1 carboxyl-domain is an ATP-repressible domain that is transferrable to other proteins PMID: 28504272
- The present study has provided novel evidence to suggest that under HFD-induced metabolic surplus, the lack of SIRT1 catalytic activity promotes release of free fatty acid from mesenteric adipose tissue (MAT) and escalate nonalcoholic fatty liver disease (NAFLD) by interfering with lipid homeostasis in both liver and MAT. PMID: 28789977
- Compared with control-diet mice, mice given an high-fat diet (HFD) for 4weeks displayed anxiolytic-like behaviors. At the same time, we observed decreased SIRT1 expression in the medial prefrontal cortex and the amygdala of HFD-fed mice. Resveratrol, an activator of SIRT1, reversed the anxiolytic-like behaviors in HFD-fed mice. PMID: 29331532
- These findings support the unifying concept that nuclear NAD(+) sensor SIRT1 broadly coordinates innate and adaptive immune reprogramming during sepsis PMID: 30069485
- Low SIRT1 expression is associated with Parkinson's disease. PMID: 29571747
- SUV39H augments intracellular reactive oxygen species levels in a SIRT1-dependent manner. PMID: 28361889
- our data strongly indicate that melatonin delays postovulatory mouse oocyte aging via a SIRT1-MnSOD-dependent pathway PMID: 29752296
- SIRT1 upregulation protects against liver injury induced by a HFD through inhibiting CD36 and the NF-kappaB pathway in mouse Kupffer cells. PMID: 29845302
- Low expression of SIRT1 is associated with Human immunodeficiency virus-associated nephropathy. PMID: 29608911
- SIRT1 levels were down-regulated in the livers in models of liver fibrosis and in activated hepatic stellate cells (HSCs) as opposed to quiescent HSCs. SIRT1 activation halted, whereas SIRT1 inhibition promoted, HSC transdifferentiation into myofibroblasts. PMID: 28970250
- Sirt1 preserves Cav1-dependent endothelial function by mitigating miR-204-mediated vascular endoplasmic reticulum stress. PMID: 28181559
- Nicotinamide riboside (NR) can protect against ethanol induced liver injuries via replenishing NAD(+), reducing oxidative stress, and activating SirT1-PGC-1a-mitochondrial biosynthesis. Our data indicate that SirT1 plays an important role in the protection of NR against lipid accumulation and mitochondrial dysfunctions induced by ethanol PMID: 29679894
- These data highlight the importance of preventing the loss of 24-OH in the brain, and of maintaining high levels of the enzyme SIRT1, in order to counteract neurodegeneration. PMID: 29883958
- the results of the present study indicated that GLP1 may be a promising target for the development of novel therapeutic strategies for HGinduced nephropathy, and may function through the activation of SIRT1 PMID: 29845208
- In lung tissues of LPS-endotoxemia, significantly increased levels of SNO-SIRT were found. Plasma nitrite and HMGB1 levels were significantly higher than those in the sham controls. PMID: 29680657
- targeted induction of the anti-inflammatory effects of SIRT1 and LCN2 may help prevent obesity-associated insulin resistance and neuroinflammation. PMID: 29634925
- PE particle-induced downregulation of SIRT1 enhances ER stress. PMID: 29775898
- During catecholamine-stimulated lipolysis, Perilipin 5 is phosphorylated by protein kinase A and forms transcriptional complexes with PGC-1alpha and SIRT1 in the nucleus. Perilipin 5 promotes PGC-1alpha co-activator function by disinhibiting SIRT1 deacetylase activity. PMID: 27554864
- findings demonstrate a new mechanism for the activation of SIRT1 under stress conditions and suggest a novel potential therapeutic target for preventing age-related diseases and extending healthspan PMID: 29133780
- SIRT1 was inhibited or knocked out in macrophages, the protection effect disappeared by treatment of inhibitor of mir-138, and the NFkappaB pathway was activated and the AKT pathway was inhibited PMID: 30157481
- the results of the present study suggest that moderate overexpression of SIRT1 (~3fold of normal level) may directly or indirectly inhibit apoptosis of OBs via the FOXO1 and betacatenin signaling pathway. PMID: 29512706
- Overexpression of miR-22 can attenuate oxidative stress injury in diabetic cardiomyopathy by upregulation of Sirt 1. PMID: 29288528
- caloric restriction rescues SIRT1 levels in transgenic Machado-Joseph disease (MJD) mice, whereas silencing SIRT1 is sufficient to prevent the beneficial effects on MJD pathology. PMID: 27165717
- the findings identify a gut-vascular axis in which Gut microbiota remotely upregulate miR-204 expression in the vessel wall. Among the genes targeted by miR-204 in the vascular wall is Sirt1, downregulation of which promotes endothelial dysfunction. PMID: 27586459
- melatonin oral supplementation may represent a new protective approach to block hypercholesterolemic liver alterations involving also a SIRT1-dependent mechanism. PMID: 29516009
- Melatonin promoted MnSOD and SIRT1 expression, which successfully ameliorated busulfan-induced SSC apoptosis caused by high concentrations of ROS and p53 PMID: 28027652
- HO-1 regulates macrophage activation via the SIRT1-p53 signaling network and regulates hepatocellular death in liver ischemia-reperfusion injury. PMID: 28842295
- miR-200a-3p promotes beta-amyloid-induced neuronal apoptosis through down-regulation of SIRT1 in Alzheimer's disease and its animal models. PMID: 29358553
- The nicotinamide adenine dinucleotide (NAD)-dependent deacetylase SIRT1 acts as an energy sensor and negatively regulates poly(A)RNA transport via deacetylating a poly(A)-binding protein, PABP1. PMID: 28756945
- L-Carnitine alleviated epithelial mesenchymal transformation-associated renal fibrosis caused by perfluorooctanesulfonate through a Sirt1- and PPARgamma-dependent mechanism. PMID: 28973641
- The data, therefore, suggest that 1,25(OH)2D3 increases glucose consumption by inducing SIRT1 activation, which in turn increases IRS1 phosphorylation and GLUT4 translocation in myotubes. PMID: 29188534
- PU.1 suppresses Sirt1 translation via transcriptional promotion of miR-34a/-29c. PMID: 29162670
- Impairment of energy sensors, SIRT1 and AMPK, in lipid induced inflamed adipocyte is regulated by Fetuin A. PMID: 29030114
- this study revealed a new mechanism wherein EPO alleviates hepatic steatosis by activating autophagy via SIRT1-dependent deacetylation of LC3. PMID: 29522896
- Retinoic acid (RA) increases the expression of hepatic Sirt1 and inhibits the expression of sterol regulatory element binding protein 1c (Srebp-1c) in WT mice in vivo and in vitro. RA-mediated molecular effects are abolished in the liver and primary hepatocytes from Sirtuin 1 (Sirt1) deficient (LKO) mice. PMID: 28667519
- thymoquinone inhibits cellular ROS generation in LPS-activated BV2 microglia. It is also suggested that activation of both AMPK and NAD(+)/SIRT1 may contribute to the anti-inflammatory, but not antioxidant activity of the compound in BV2 microglia. PMID: 28551846
- SIRT1 signaling is important for alleviation of sepsis-induced myocardial injury and for enhancement of the protective effect of a liver X receptor agonist. PMID: 28993270
- reduction of Sirt1 activity restores adipogenesis in Sirt7(-/-) adipocytes in vitro and in vivo PMID: 28923965
- These results support previous work demonstrating that induction of SIRT1 in skeletal muscle, either at birth or in adulthood, does not impact muscle insulin action or mitochondrial function. PMID: 28544355
- we identified that Sirtuin 1 (SIRT1), a deacetylase that suppresses FoxO1 acetylation in granulosa cell (GCs), was downregulated by miR-181a and reversed the promoting effects of H2O2 and miR-181a on FoxO1 acetylation and GC apoptosis. PMID: 28981116
- the interaction of TIM4TIM1 was found to promote Th2cell proliferation through enhancing SIRT1 expression in mice with nasal allergic rhinitis. PMID: 28949386
- retinal vasculature from diabetic Sirt1 mice did not present any increase in the number of apoptotic cells or degenerative capillaries or decrease in vascular density. Diabetic Sirt1 mice were also protected from mitochondrial damage and had normal electroretinography responses and ganglion cell layer thickness. PMID: 29311218
- CKII-SIRT1-SM22alpha acts in a loop to reinforce the expression of SM22alpha, which limits the inflammatory response in vascular smooth muscle cells. PMID: 28419207
- SIRT1 may increase neuron function and resilience against Alzheimer's disease PMID: 27614878
- Regulation of stem cell aging by SIRT1 occurs via linking metabolic signaling to epigenetic modifications. (Review) PMID: 28392411
- Data show that 66-kDa Src homology 2 domain-containing protein (p66Shc) is acetylated under high glucose conditions and is deacetylated by Sirtuin1 lysine deacetylase (Sirt1) on lysine 81. PMID: 28137876
顯示更多
收起更多
-
亞細(xì)胞定位:Nucleus, PML body. Cytoplasm. Nucleus.; [SirtT1 75 kDa fragment]: Cytoplasm. Mitochondrion.
-
蛋白家族:Sirtuin family, Class I subfamily
-
組織特異性:Widely expressed. Weakly expressed in liver and skeletal muscle.
-
數(shù)據(jù)庫鏈接:
Most popular with customers
-
Recombinant Human Cell adhesion molecule 1 (CADM1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Myosin regulatory light polypeptide 9 (MYL9) (Active)
Express system: Yeast
Species: Homo sapiens (Human)
-
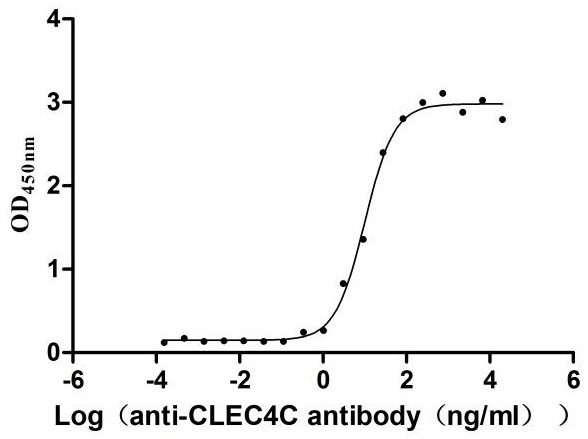
Recombinant Human C-type lectin domain family 4 member C (CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
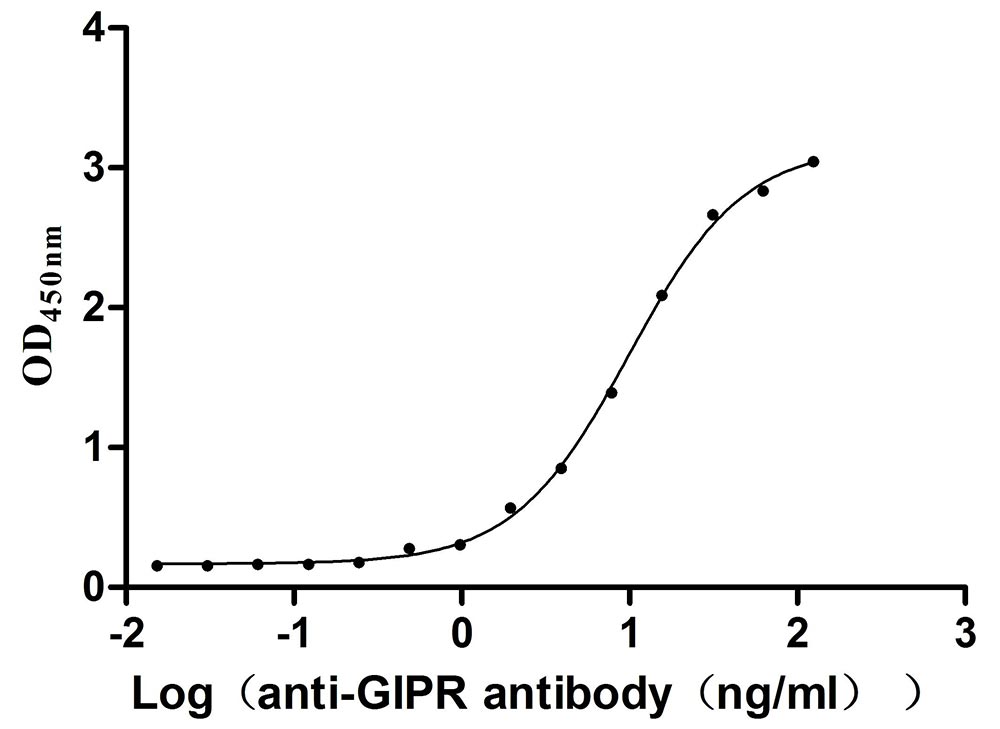
Recombinant Mouse Gastric inhibitory polypeptide receptor (Gipr), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Human Oncostatin-M (OSM), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse CUB domain-containing protein 1 (Cdcp1), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Rat Gastric inhibitory polypeptide receptor (Gipr), partial (Active)
Express system: Mammalian cell
Species: Rattus norvegicus (Rat)
-
Recombinant Macaca fascicularis CUB domain containing protein 1 (CDCP1), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)