Recombinant Rat Islet amyloid polypeptide (Iapp)
-
中文名稱:大鼠Iapp重組蛋白
-
貨號(hào):CSB-YP010931RA
-
規(guī)格:
-
來(lái)源:Yeast
-
其他:
-
中文名稱:大鼠Iapp重組蛋白
-
貨號(hào):CSB-EP010931RA
-
規(guī)格:
-
來(lái)源:E.coli
-
其他:
-
中文名稱:大鼠Iapp重組蛋白
-
貨號(hào):CSB-EP010931RA-B
-
規(guī)格:
-
來(lái)源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:大鼠Iapp重組蛋白
-
貨號(hào):CSB-BP010931RA
-
規(guī)格:
-
來(lái)源:Baculovirus
-
其他:
-
中文名稱:大鼠Iapp重組蛋白
-
貨號(hào):CSB-MP010931RA
-
規(guī)格:
-
來(lái)源:Mammalian cell
-
其他:
產(chǎn)品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
別名:IappIslet amyloid polypeptide; Amylin; Diabetes-associated peptide; DAP
-
種屬:Rattus norvegicus (Rat)
-
蛋白長(zhǎng)度:Cytoplasmic domain
-
表達(dá)區(qū)域:38-74
-
氨基酸序列KCN TATCATQRLA NFLVRSSNNL GPVLPPTNVG SNTY
-
蛋白標(biāo)簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產(chǎn)品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復(fù)溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲(chǔ)存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項(xiàng):Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶點(diǎn)詳情
-
功能:Selectively inhibits insulin-stimulated glucose utilization and glycogen deposition in muscle, while not affecting adipocyte glucose metabolism.
-
基因功能參考文獻(xiàn):
- IAPP role in the pancreatic islet cell damage in the Type 2 diabetes mellitus. PMID: 29523142
- this study reinforces the notion that IAPP amyloidogenesis is governed by precise intermolecular interactions involving specific Asn side chains. PMID: 28665109
- C4BP protects beta-cells from IAPP cytotoxicity by modulating IAPP fibril formation extracellularly and also, after uptake by the cells, by enhancing cholesterol synthesis. PMID: 27566545
- ventromedial hypothalamus amylin signaling is required for full leptin signaling and protection from high fat diet induced obesity PMID: 26676252
- Secondary Structure of Rat and Human Amylin PMID: 26221949
- cross-seeding assemblies between hIAPP and rIAPP oligomers PMID: 25706385
- Inhibition of IAPP aggregation by insulin depends on the insulin oligomeric state regulated by zinc ion concentration PMID: 25649462
- The data indicate minimal membrane disruption by both human and rat-IAPP20-29 when compared to the full-length IAPP peptide. PMID: 23493863
- Amylin receptors are located mostly on presynaptic glutamatergic terminals connecting to area postrema neurons not displaying hyperpolarization-activated cation current. Amylin concentrations can increase glutamate release enough to cause cell firing. PMID: 23219578
- C4BP binds to IAPP thereby limiting complement activation and may be enhancing formation of IAPP fibrils from cytotoxic oligomers PMID: 22334700
- co-expression of calcitonin receptor(a) with rRAMP1 or 3 produces functional AMY receptor subtypes in rats, which display equivalent potency for rAmy and raCGRP PMID: 22014233
- at 8 h after mixing, rat amylin blocks the N-terminal beta-sheet of fibrils. At 24 h after mixing, rat amylin blocks neither beta-sheet and forms its own beta-sheet PMID: 22522254
- Data show that the multiplicity in islet amyloid polypeptide (rIAPP) assembly structures indicates the polydispersity of the rIAPP(8-37) beta-like motifs. PMID: 22106265
- The results implicate amylin in the control of maternal adaptations, possibly exerting its actions on maternal behaviors via amylin receptors present in brain regions to which preoptic neurons project. PMID: 21965599
- hIAPP overexpression inhibits insulin and IAPP secretion in response to glucose affecting the activity of K(ATP) channels PMID: 21984830
- an actin-mediated and cholesterol-dependent mechanism for selective uptake and clearance of amylin oligomers, impairment of which greatly potentiates amylin toxicity. PMID: 21865171
- The study is comparing the structural properties of human and rat islet amyloid polypeptide by MD computer simulations at 310 and 330 K and shows the effect of the disulfide bridge on the conformation of Iapp. PMID: 21266296
- Data show that IAPP oligomeric species that arise through stochastic nucleation on membranes and result in disruption of the lipid bilayer. PMID: 21606325
- Ca2+ entry via L-type Ca2+ channels, activation of PKC and Ca2+ release from sarcoplasmic reticulum through ryanodine-sensitive Ca2+ channels may be involved in amylin's positive inotropic effect. PMID: 21138812
- Rats centrally infused with amylin had lower body weight gain controls independent of whether prior body weight was decreased by fasting, increased by voluntary overfeeding or unmanipulated. PMID: 20416330
- Data indicate that basal insulin, leptin, ghrelin, and amylin do not encode AT mass in rats dynamically regulating BW and adiposity during recovery from OW. PMID: 20668029
- we present detailed solution structures of rat amylin; the similarity of rat and human amylin sequences near the N-terminus suggests that human amylin might also exhibit a similar a-helical secondary structure PMID: 20141758
- This His/Arg-18 mutation results in reduced affinity binding of human IAPP to insulin in comparison to rat IAPP. PMID: 19146426
- Inhibition of human IAPP amyloid formation by rat IAPP can be rationalized by a model that postulates formation of an early helical intermediate during amyloid formation where the helical region is localized to the N-terminal region of IAPP. PMID: 20028124
- these newly observed differences between human and rat amylin in solution and their possible relation to aggregation and to the physiological function of amylin binding to the calcitonin receptor PMID: 19948124
- Data show that the three specific proline residues in human and rat amylin play a dominant negative role in fibril formation. PMID: 12589759
- conformational preferences of hIAPP(20-29) in membrane-mimicking environments PMID: 12717720
- Feeding-induced amylin release activates area postrema neurons projecting to relay stations known to transmit meal-related signals to the forebrain. Activation of this pathway seems to coincide with an inhibition of lateral hypothalamic area neurons. PMID: 12958059
- amylin has a role in evoking a JNK1-mediated apoptotic pathway, which is partially dependent and partially independent of caspase-8, and in which caspase-3 acts as a common downstream effector PMID: 14532296
- These results indicate that amylin is positively regulated by cyclic AMP and protein kinase A through the transcription factors HNF-1 and NFY. PMID: 14615061
- results support the hypothesis that endogenous amylin plays an essential role in reducing meal size and increasing the postmeal interval of satiety PMID: 15130879
- found that amylin increased GSK3alpha activity, which in turn led to increased phosphorylation of glycogen synthase and decreased glycogen synthesis de novo in rat soleus muscle PMID: 15572200
- findings are consistent with the hypothesis that membrane-active pre-fibrillar IAPP oligomers insert into beta cell membranes in NIDDM PMID: 15710403
- analysis of pro-islet amyloid polypeptide processing in the constitutive and regulated secretory pathways of beta cells PMID: 15802374
- conditions that promote weakly stable alpha-helical conformations may promote IAPP aggregation PMID: 16142909
- Data show that GABAA receptor antagonists prevent abnormalities in leptin, insulin and amylin actions on paraventricular hypothalamic neurons of overweight rats. PMID: 16553787
- The data suggests that both KNT and KNTvd4(-) participate in microtubule-dependent secretion of amylin in islet beta-cells. PMID: 16890462
- The inability of IAPP to self assemble can be ascribed, in part, to several slowly exchanging conformations evident as multiple chemical shift assignments in the immediate vicinity of three proline residues residing outside of this helical region. PMID: 17123962
- Amylin did not influence energy expenditure or respiratory quotient in normal or food-restricted rats. PMID: 17428511
- Results indicate that the lipostatic signals leptin and insulin may synergize with amylin to reduce food intake. PMID: 17481674
- amylin effectively reduced food intake in ad libitum chow fed rats despite the low level of amylin-induced c-Fos expression in the area postrema PMID: 17703089
- Central and peripheral administration of amylin induces energy expenditure in anesthetized rats. PMID: 18346817
- amylin agonism restores leptin responsiveness in diet-induced obesity PMID: 18458326
- Rat amylin does not share neurotoxic properties with Abeta42. PMID: 18486611
- Amylin/leptin interact synergistically to reduce body weight and adiposity in diet-induced obese rodents through a number of anorexigenic and metabolic effects. PMID: 18669592
- NMR structures of the human and rat IAPP(1-19) peptides in DPC micelles PMID: 18989932
- the high-resolution structure of rat IAPP in the membrane-mimicking detergent micelles composed of dodecylphosphocholine was solved. PMID: 19456151
- Amylin is expressed in rat placenta and regulated by nutritional status. PMID: 19554505
- This studt indicted that the expression of amylin in the mammalian brain and described its distribution in the preoptic area of the hypothalamus. Our data also indicate that the level of amylin mRNA is dramatically induced in mother rats PMID: 19811608
顯示更多
收起更多
-
亞細(xì)胞定位:Secreted.
-
蛋白家族:Calcitonin family
-
組織特異性:Abundant in the islets of Langerhans but is not present in the brain or seven other tissues examined.
-
數(shù)據(jù)庫(kù)鏈接:
Most popular with customers
-
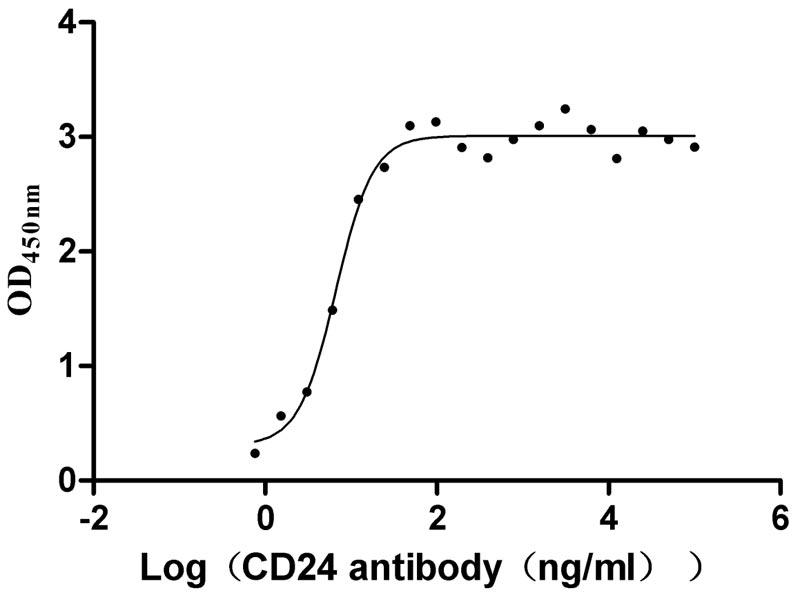
Recombinant Human Signal transducer CD24 (CD24)-Nanoparticle (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human B-lymphocyte antigen CD20 (MS4A1)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse Tyrosine-protein kinase Mer (Mertk), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Human Urokinase-type plasminogen activator(PLAU) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human C-C chemokine receptor type 5 (CCR5)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)