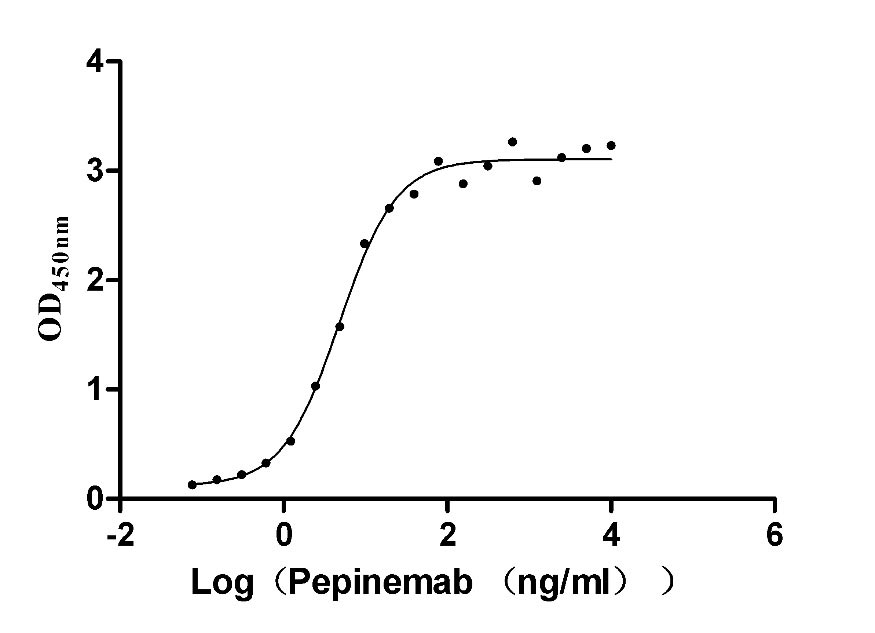
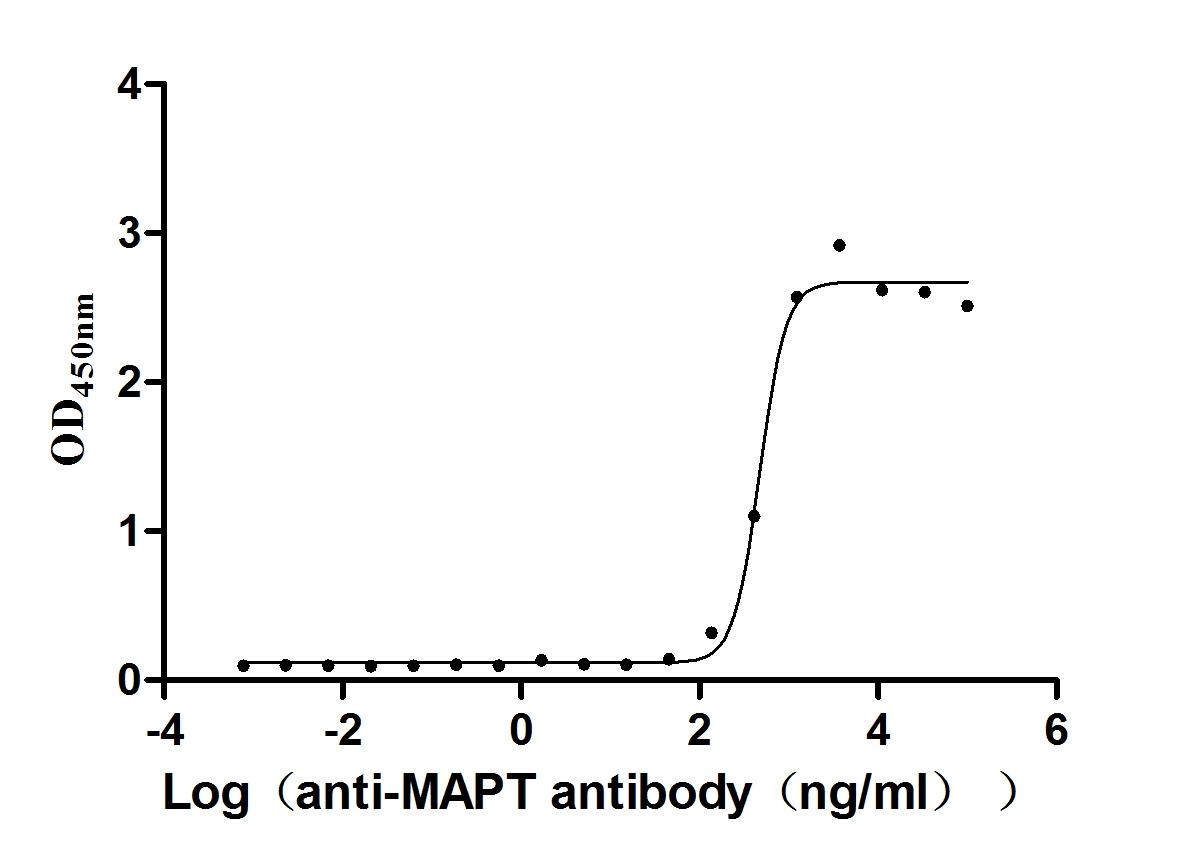
Recombinant Bovine Cytochrome c oxidase subunit 4 isoform 1, mitochondrial (COX4I1)
-
中文名稱:Recombinant Bovine Cytochrome c oxidase subunit 4 isoform 1, mitochondrial(COX4I1)
-
貨號:CSB-CF005832BO
-
規(guī)格:
-
來源:in vitro E.coli expression system
-
其他:
產(chǎn)品詳情
-
基因名:
-
Uniprot No.:
-
別名:COX4I1; COX4; Cytochrome c oxidase subunit 4 isoform 1, mitochondrial; Cytochrome c oxidase polypeptide IV; Cytochrome c oxidase subunit IV isoform 1; COX IV-1
-
種屬:Bos taurus (Bovine)
-
蛋白長度:Full Length of Mature Protein
-
表達(dá)區(qū)域:23-169
-
氨基酸序列AHGSVVKSEDYALPSYVDRRDYPLPDVAHVKNLSASQKALKEKEKASWSSLSIDEKVELY RLKFKESFAEMNRSTNEWKTVVGAAMFFIGFTALLLIWEKHYVYGPIPHTFEEEWVAKQT KRMLDMKVAPIQGFSAKWDYDKNEWKK
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白標(biāo)簽:N-terminal 10xHis-tagged
-
產(chǎn)品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
緩沖液:Lyophilized from Tris/PBS-based buffer, 6% Trehalose, pH 8.0
-
儲存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質(zhì)期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相關(guān)產(chǎn)品
靶點詳情
-
功能:Component of the cytochrome c oxidase, the last enzyme in the mitochondrial electron transport chain which drives oxidative phosphorylation. The respiratory chain contains 3 multisubunit complexes succinate dehydrogenase (complex II, CII), ubiquinol-cytochrome c oxidoreductase (cytochrome b-c1 complex, complex III, CIII) and cytochrome c oxidase (complex IV, CIV), that cooperate to transfer electrons derived from NADH and succinate to molecular oxygen, creating an electrochemical gradient over the inner membrane that drives transmembrane transport and the ATP synthase. Cytochrome c oxidase is the component of the respiratory chain that catalyzes the reduction of oxygen to water. Electrons originating from reduced cytochrome c in the intermembrane space (IMS) are transferred via the dinuclear copper A center (CU(A)) of subunit 2 and heme A of subunbit 1 to the active site in subunit 1, a binuclear center (BNC) formed by heme A3 and copper B (CU(B)). The BNC reduces molecular oxygen to 2 water molecules using 4 electrons from cytochrome c in the IMS and 4 protons from the mitochondrial matrix.
-
基因功能參考文獻(xiàn):
- Structure of bovine cytochrome c oxidase crystallized at a neutral pH has been reported. PMID: 28695851
- Studies indicate that the patterns of charge translocation of cytochrome c oxidase coupled to transfer of the 3rd and 4th electrons are very similar. PMID: 21889506
- Studies indicate that time-resolved resonance Raman (RR) spectroscopy of whole mitochondria identified a band at 571cm(-1) arising from the oxygenated intermediate at Deltat=0.4, 0.6 and 1.4ms. PMID: 22172733
- Studies indicate that mutational amino acid replacement in proton channels, at the negative (N) side of membrane-inserted prokaryotic aa(3) oxidases, as well as Zn(2+) binding at this site in the bovine oxidase, uncouples proton pumping. PMID: 22100820
- Studies indicate that nitric oxide (NO) binding to reduced ba(3) and bovine cytochrome aa3. PMID: 22201543
- Studies indicate that X-ray structure of heart cytochrome c oxidase (CcO) suggest that O(2) molecules are transiently trapped at the Cu(B) site before binding to Fe(a3)(2+) to provide O(2)(-). PMID: 22236806
- Studies indicate that photoexcitation of Ru (II) to Ru(II*) leads to rapid electron transfer to the ferric heme group in cytochrome c (Cc), followed by electron transfer to Cu(A) in cytochrome c oxidase (CcO) with a rate constant of 60,000s(-1). PMID: 21939635
- Studies suggest that the master-equation models address the question of how pumping can be achieved in a system in which all reaction steps are reversible. PMID: 21946020
- Studies suggest for the His291 model of proton pumping in cytochrome c oxidase (CcO). PMID: 22086149
顯示更多
收起更多
-
亞細(xì)胞定位:Mitochondrion inner membrane; Single-pass membrane protein.
-
蛋白家族:Cytochrome c oxidase IV family
-
數(shù)據(jù)庫鏈接:
Most popular with customers
-
Recombinant Human Tumor necrosis factor ligand superfamily member 8 (TNFSF8), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse Semaphorin-4D (Sema4d), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Human Semaphorin-4D (SEMA4D), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human IGF-like family receptor 1 (IGFLR1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse Microtubule-associated protein tau (Mapt) (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Macaca fascicularis CD44 antigen (CD44), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Human Desmoglein-2 (DSG2), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)